Uses of aminosalicylic acid derivative as neuroprotective agent
A technology of aminosalicylic acid and derivatives, applied in the field of aminosalicylic acid derivatives and neuroprotective agents, to achieve strong cell damage, good neuroprotective effect, and good therapeutic activity for stroke
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
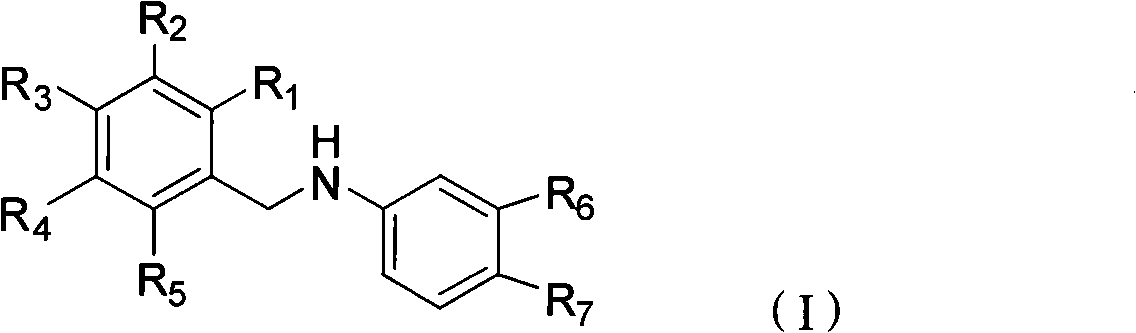
[0026] Example 15-(5-chloro-2-hydroxybenzylamino)-2-hydroxybenzoic acid (JX-1)
[0027] Dissolve 1.57g (0.01mol) of 5-chloro-2-hydroxybenzaldehyde in 20ml of absolute ethanol, 1.53g (0.01mol) of 5-aminosalicylic acid in 40ml of absolute ethanol, 5ml of water and 1ml of concentrated hydrochloric acid In the mixed solvent, mix the two, reflux for 30 minutes, cool and precipitate the solid, filter, add 20ml of absolute ethanol to the filter cake, add 1.5g of sodium borohydride at 0-5°C, after the addition is complete, stir for 0.5 hours, and reflux for 30 minutes. Cool, adjust the pH to 1-2 with concentrated hydrochloric acid, filter, add 100ml of water, stir for 30 minutes, and filter to obtain the target compound.
Embodiment 25
[0028] Example 25-(5-bromo-2-hydroxybenzylamino)-2-hydroxybenzoic acid (JX-2)
[0029] Dissolve 2.01g (0.01mol) of 5-bromo-2-hydroxybenzaldehyde in 20ml of absolute ethanol, 1.53g (0.01mol) of 5-aminosalicylic acid in 40ml of absolute ethanol, 5ml of water and 1ml of concentrated hydrochloric acid In the mixed solvent, mix the two, reflux for 30 minutes, cool and precipitate the solid, filter, add 20ml of absolute ethanol to the filter cake, add 1.5g of sodium borohydride at 0-5°C, after the addition is complete, stir for 0.5 hours, and reflux for 30 minutes. Cool, adjust the pH to 1-2 with concentrated hydrochloric acid, filter, add 100ml of water, stir for 30 minutes, and filter to obtain the target compound.
[0030] 13 C-NMR (d-DMSO) δ: 40.34, 110.03, 111.79, 112.60, 111.96, 117.45, 122.00, 128.3, 129.70, 130.00, 140.51, 153.07, 154.35, 171.81
Embodiment 3
[0031] Example 3 5-(3,5-dichloro-2-hydroxybenzylamino)-2-hydroxybenzoic acid (JX-3)
[0032] Dissolve 1.91g (0.01mol) of 3,5-dichloro-2-hydroxybenzaldehyde in 20ml of absolute ethanol, 1.53g (0.01mol) of 5-aminosalicylic acid in 40ml of absolute ethanol, 5ml of water and 1ml In the mixed solvent of concentrated hydrochloric acid, mix the two, reflux for 30 minutes, cool and precipitate the solid, filter, add 20ml of absolute ethanol to the filter cake, add 1.5g of sodium borohydride at 0-5°C, complete the addition, stir for 0.5 hours, and reflux After cooling for 30 minutes, adjust the pH to 1-2 with concentrated hydrochloric acid, filter, add 100ml of water, stir for 30 minutes, and filter to obtain the target compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com