Process for the preparation of imatinib and intermediates thereof
A technology of phenyl and compound, which is used in the field of preparation of imatinib and its intermediates, which can solve problems such as time-consuming, unsuitable for industrial applications, and no economic advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
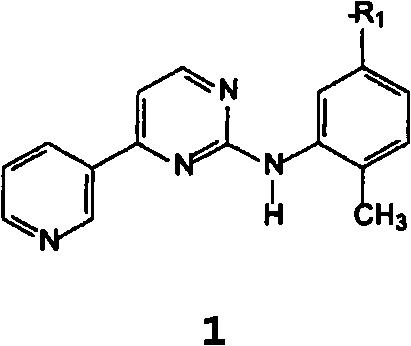
[0195] Example 1: 4-methyl-N3-[4-(3-pyridyl)-2-pyrimidinyl]-1,3-phenylenediamine 8
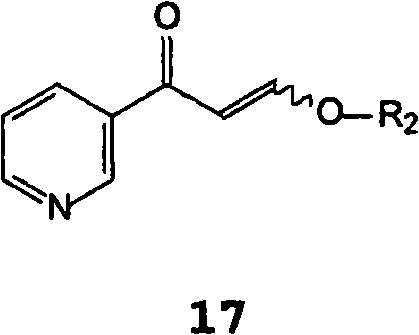
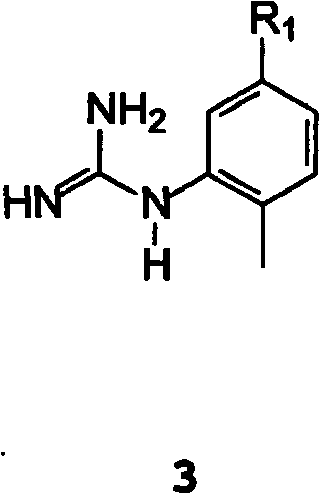
[0196] Suspend 16 g of the sodium salt of β-oxo-3-pyridinepropanal with an HPLC purity of 99% (A%) and a salt content (residue on ignition) of 25% in 115 mL of n-butanol under an inert atmosphere and 11.7 g (2-methyl-5-aminophenyl)guanidine. 9 mL of acetic acid was added, and the mixture was stirred at room temperature for 1 hour. 6 g of potassium hydroxide were added in portions and the mixture was refluxed for 18 hours, removing water with a Dean Stark apparatus. Once the conversion was complete, the suspension was cooled and the organic layer was washed with water. The organic layer was concentrated to a small volume and toluene was added. Filtration of the precipitate yielded 15.5 g of product with 99.2% HPLC purity (A%) under drying, by LC-MS and 1 H-NMR for identification.
[0197] LC-MS: [M+1] + =278.
[0198] 1 H-NMR (300MHz, DMSO-d 6 ): δ(ppm)2.02(s, 3H); 4.85(s, 2H); 6.31(d,...
Embodiment 2
[0199] Example 2: 1-(5-amino-2-methylphenyl)-3-[(3-oxo-3-(3-pyryl)-1-prop-1-enyl)guanidine 22
[0200] Suspend 10 g of the sodium salt of β-oxo-3-pyridinepropionaldehyde with a HPLC purity of 99% (A%) and a salt content (residue on ignition) of 25% in 80 mL of isopropanol under an inert atmosphere . 24 mL of 15% isopropanol hydrochloride solution was added, and the mixture was stirred at room temperature for 1 hour. 7 g of (2-methyl-5-aminophenyl)guanidine were added in portions, and the mixture was stirred at room temperature for 12 hours. Once the conversion was complete, the precipitate was filtered and dried to yield 13.5 g of product with 98% HPLC purity (A%) and 30% salt content (residue on ignition), identified by LC-MS.
[0201] LC-MS: [M+1] + =296.
Embodiment 3
[0202] Example 3: N-(2-methyl-5-nitrophenyl)-4-(3-pyridyl)-2-pyrimidinamine 1 (R 1 = NO 2 )
[0203] Under an inert atmosphere, in 50 mL of toluene, 5 g of sodium salt of β-oxo-3-pyridinepropanal and 8 mL of isopropanol hydrochloride. 3.7 g of (2-methyl-5-nitrophenyl)guanidine were added, and the mixture was stirred at room temperature for 1 hour. The mixture was refluxed for 18 hours and the water was removed using a Dean Stark apparatus. Once the conversion is complete, the suspension is cooled to 10 °C and the precipitate is filtered; the precipitate is triturated in warm water, which yields 2.5 g of product with 96% HPLC purity (A%) upon filtration and drying by GC-MS for identification.
[0204] MS m / e (int.rel.): 307 (M+) (100); 292 (76); 260 (63); 246 (38).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com