Convenient and quick method for preparing high-purity imatinib and mesylate thereof
A technology for imatinib and benzoic acid, which is applied in the field of synthetic methods of imatinib and its mesylate, can solve the problem of easy generation of demethyl imatinib impurities, many impurities produced by the reaction, difficult to remove and Purification and other issues to achieve complete transformation, product quality improvement, and good quality results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] 1. Preparation of imatinib
[0035] Reaction equation:
[0036]
Embodiment 1
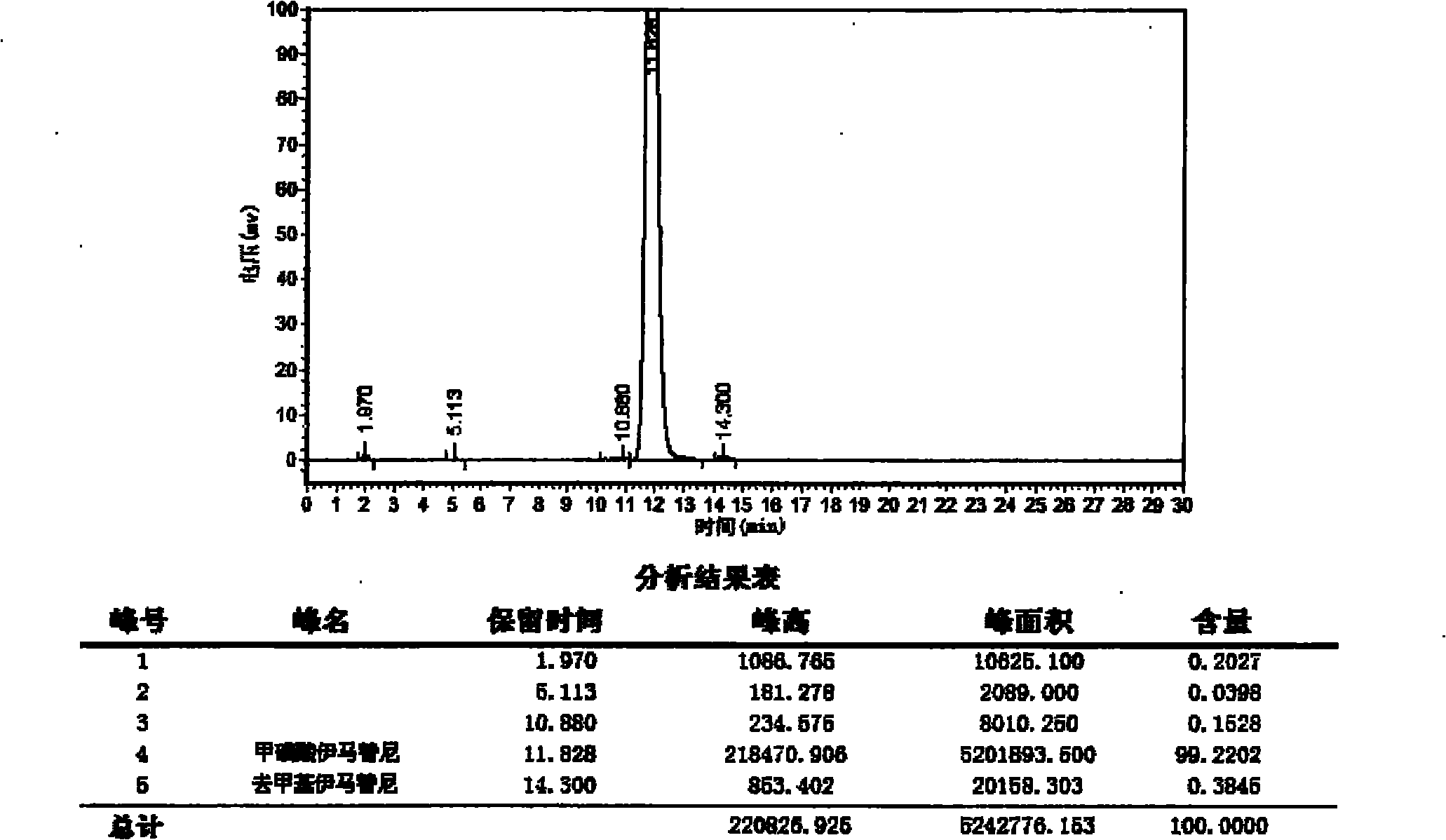
[0038] Add 4-(4-methylpiperazine methyl)-benzoic acid dihydrochloride (122g, 0.397mol) in the four-necked flask, dichloromethane (1000ml), control room temperature dropwise diisopropylethylamine ( 138ml, 0.792mol), add and keep warm for 1 hour. Add N-(5-amino-2-methylphenyl)-4-(3-pyridine)-pyrimidinamine (100g, 0.361mol), HBTU (150g, 0.396mol) and diisopropylethylamine (75ml, 0.431mol). Add and keep warm overnight. Dilute aqueous sodium hydroxide (500ml) was added at room temperature and stirred for 1 hour. Suction filtration, rinse with appropriate amount of water and ethyl acetate to obtain filter cake 1.
[0039] The mother liquor was separated into phases, and the organic phase was separated, dried with anhydrous sodium sulfate, and filtered with suction. The filtrate was concentrated to dryness under reduced pressure to obtain a light yellow solid, which was crystallized by adding ethyl acetate and stirring. Suction filtration and rinsing with an appropriate amount o...
Embodiment 2
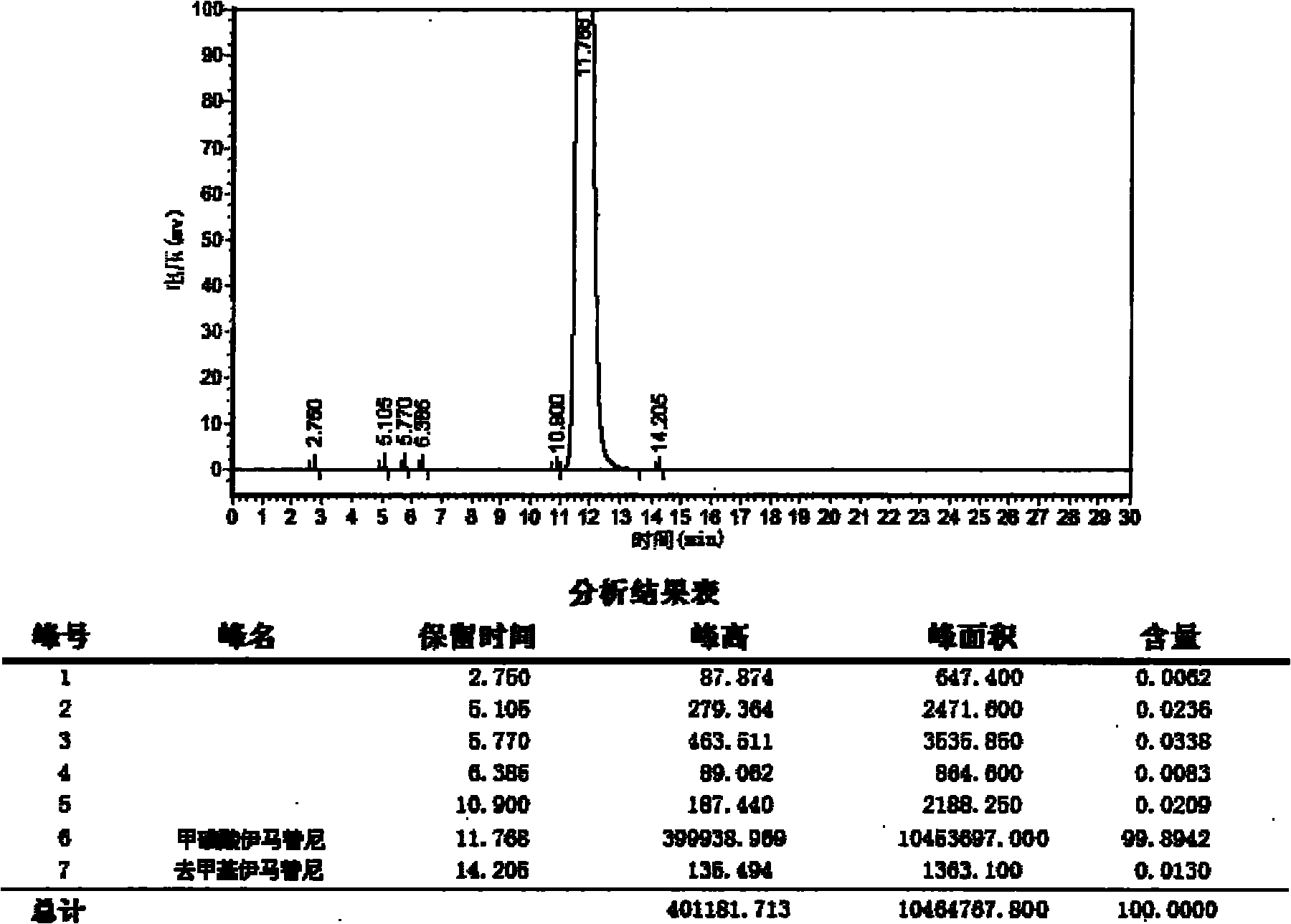
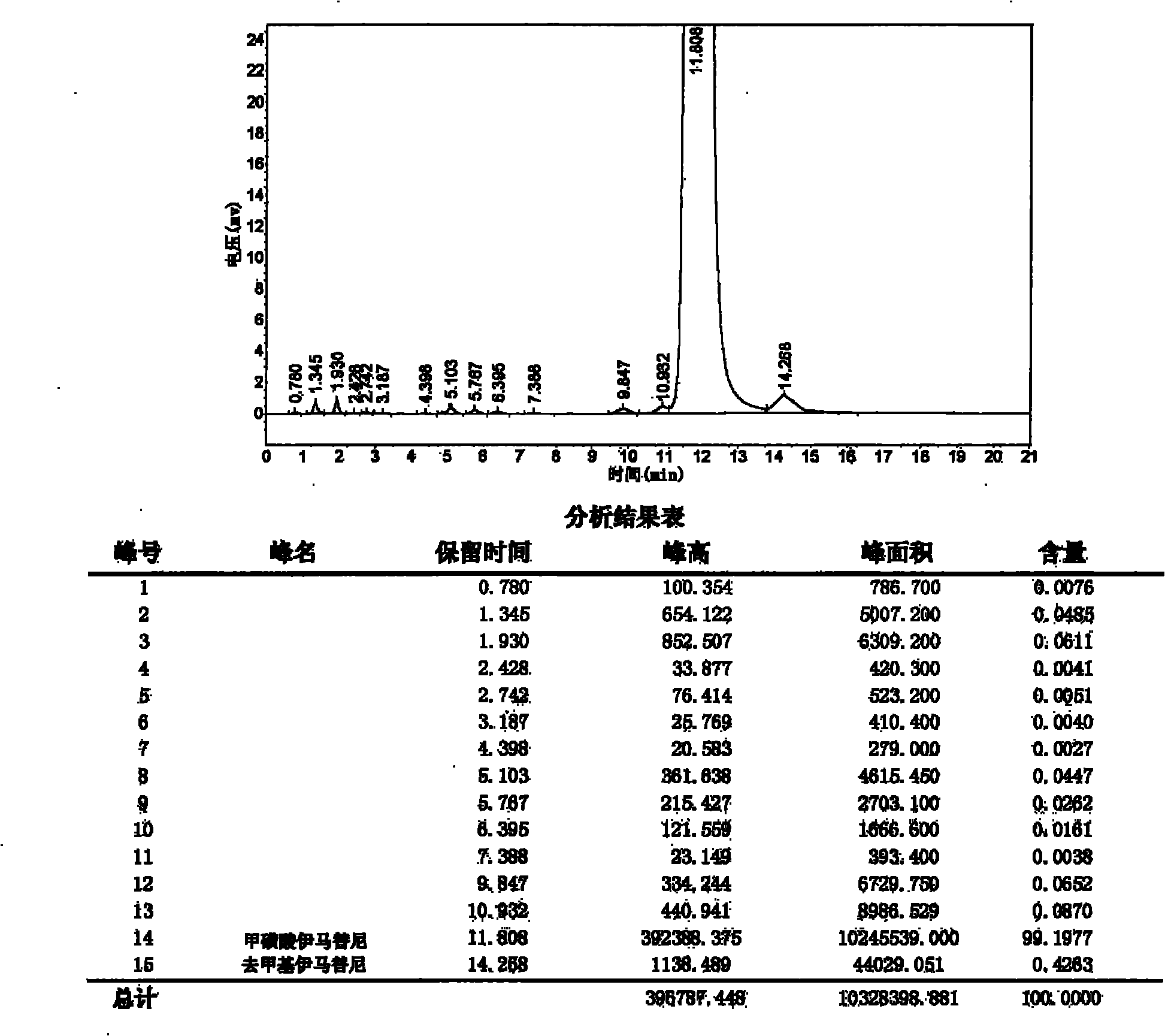
[0041] Add 4-(4-methylpiperazinemethyl)-benzoic acid dihydrochloride (24.4g, 0.079mol), DMF (120ml) in a four-neck flask, and add DIPEA (28ml, 0.161mol) dropwise at room temperature, After the addition, keep stirring for 30 minutes. N-(5-Amino-2-methylphenyl)-4-(3-pyridine)-pyrimidinamine (20 g, 0.072 mol), HCTU (32.7 g, 0.079 mol) and DIPEA (15 ml, 0.086 mol) were added. After the addition, the reaction was incubated for 8 hours. After the reaction is complete, add water and stir for 30 minutes, filter with suction, wash the filter cake with ethyl acetate, and dry at 50°C. 33.1 g (purity: 97.8%) of the target object was obtained as a pale yellow solid. "Desmethylimatinib" was not detected. Yield: 93.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com