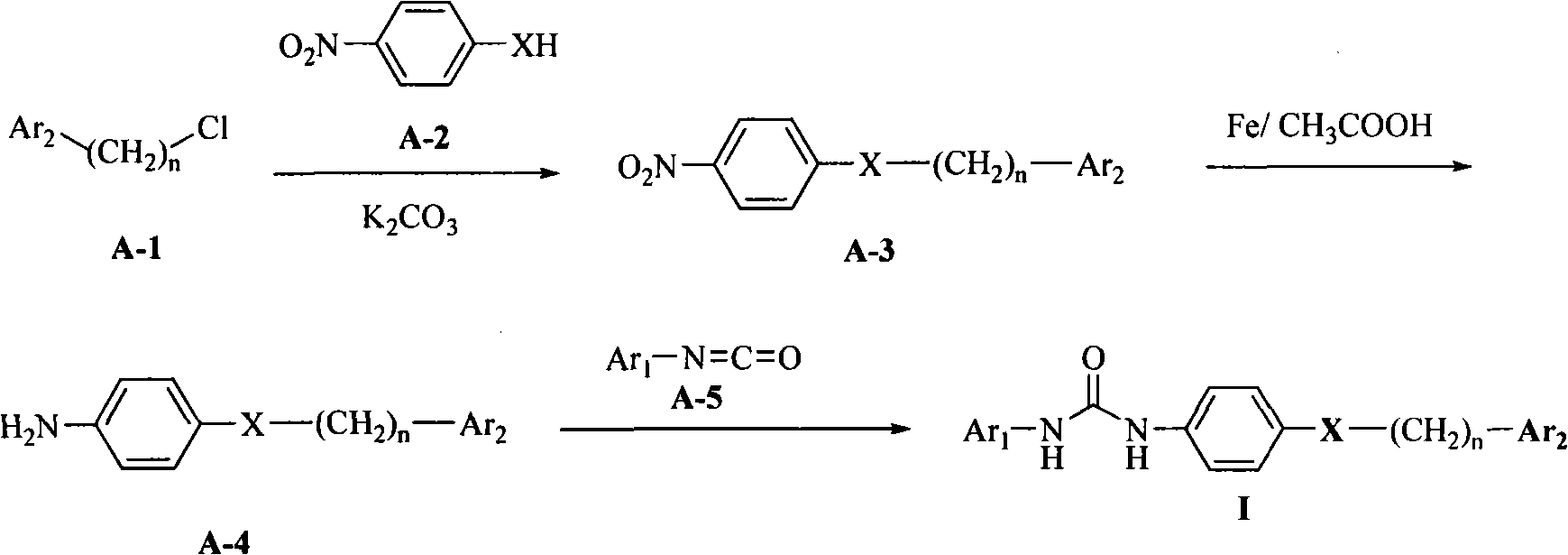
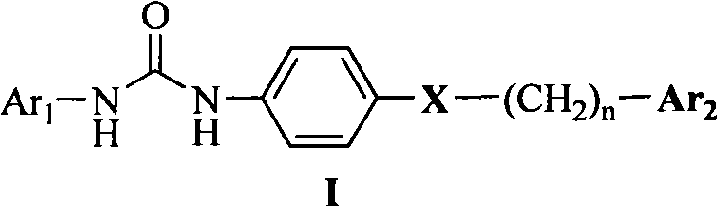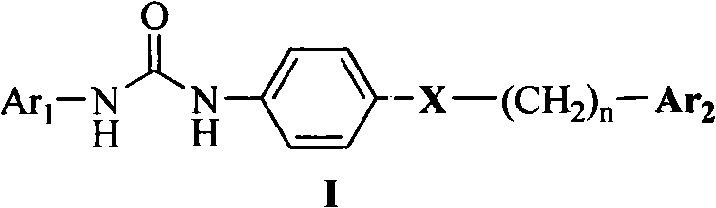Diarylurea derivatives and application thereof used for preparing anti-neoplastic medicament
A technology of derivatives and heteroaryl groups, which can be used in anti-tumor drugs, drug combinations, medical preparations containing active ingredients, etc., and can solve problems such as insufficient inhibition of tumor development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1: 1-[4-[(8-methyl-4-oxo-4H-pyrido[1,2-a]pyrimidin-2-yl)methoxy]phenyl]-3-(2 , 6-difluorophenyl) urea methanesulfonate;
[0103] Step A: Preparation of 2-chloromethyl-8-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
[0104] Add 38g (0.35mol) of 2-amino-4-picoline and 250g of polyphosphoric acid into a 1000ml three-necked flask, and heat to 45°C. Under stirring, 55 ml (0.43 mol) of ethyl 4-chloroacetoacetate was added dropwise, and after the drop was completed, the temperature was raised to 125° C. for about 2 hours. Cool to 50°C after the reaction is complete, then pour into a large amount of ice water, CHCl 3 Extract, wash with water, and dry over anhydrous sodium sulfate. Evaporate to dryness to obtain 60.0 g of off-white solid, yield: 81.4%, MS: 209 (M+1).
[0105] Step B: Preparation of 8-methyl-2-[(4-nitrophenoxy)methyl]-4H-pyrido[1,2-a]pyrimidin-4-one
[0106] Dissolve 27g (0.129mol) of 2-chloromethyl-8-methyl-4H-pyrido[1,2-a]pyrimidin-4-one in 200ml of DMF,...
Embodiment 2
[0115] 1-[4-[(8-methyl-4-oxo-4H-pyrido[1,2-a]pyrimidin-2-yl)methoxy]phenyl]-3-(2,4-di Methylphenyl)urea methanesulfonate;
[0116] MS: 429(M+1)
[0117] 1 H-NMR (DMSO-d 6 )δ(ppm): 8.98(s, 1H), 8.89(s, 1H), 7.85(s, 1H), 7.68(s, 1H), 7.61(s, 1H), 7.48(s, 1H), 7.42( s, 2H), 6.96~7.01 (t, J 1 =9Hz,J 2 =6.6Hz, 4H), 6.55(s, 1H), 5.13(s, 2H), 2.57(s, 3H), 2.20(s, 3H), 2.18(s, 3H).
Embodiment 3
[0119] 1-[4-[(4-oxo-4H-pyrido[1,2-a]pyrimidin-2-yl)methoxy]phenyl]-3-(2,4-dimethylphenyl) Urea mesylate;
[0120] MS: 415(M+1);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com