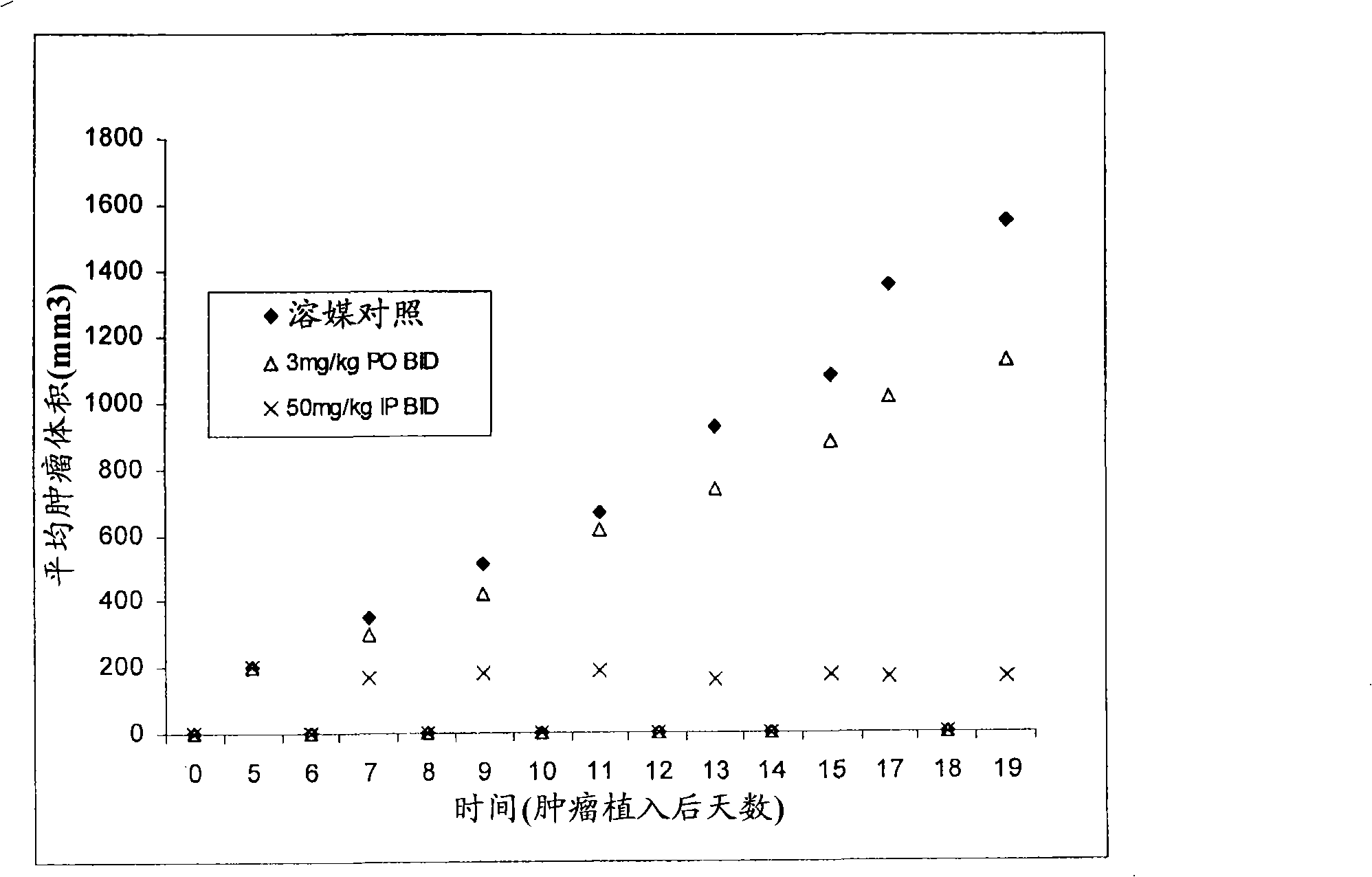
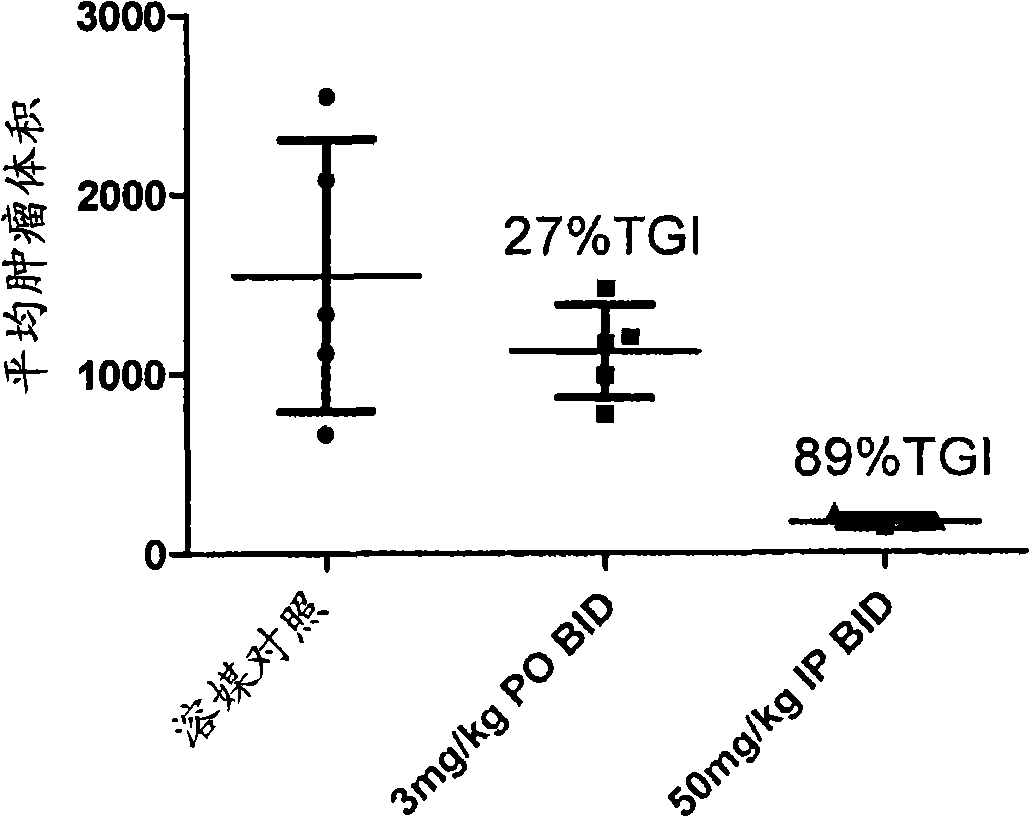
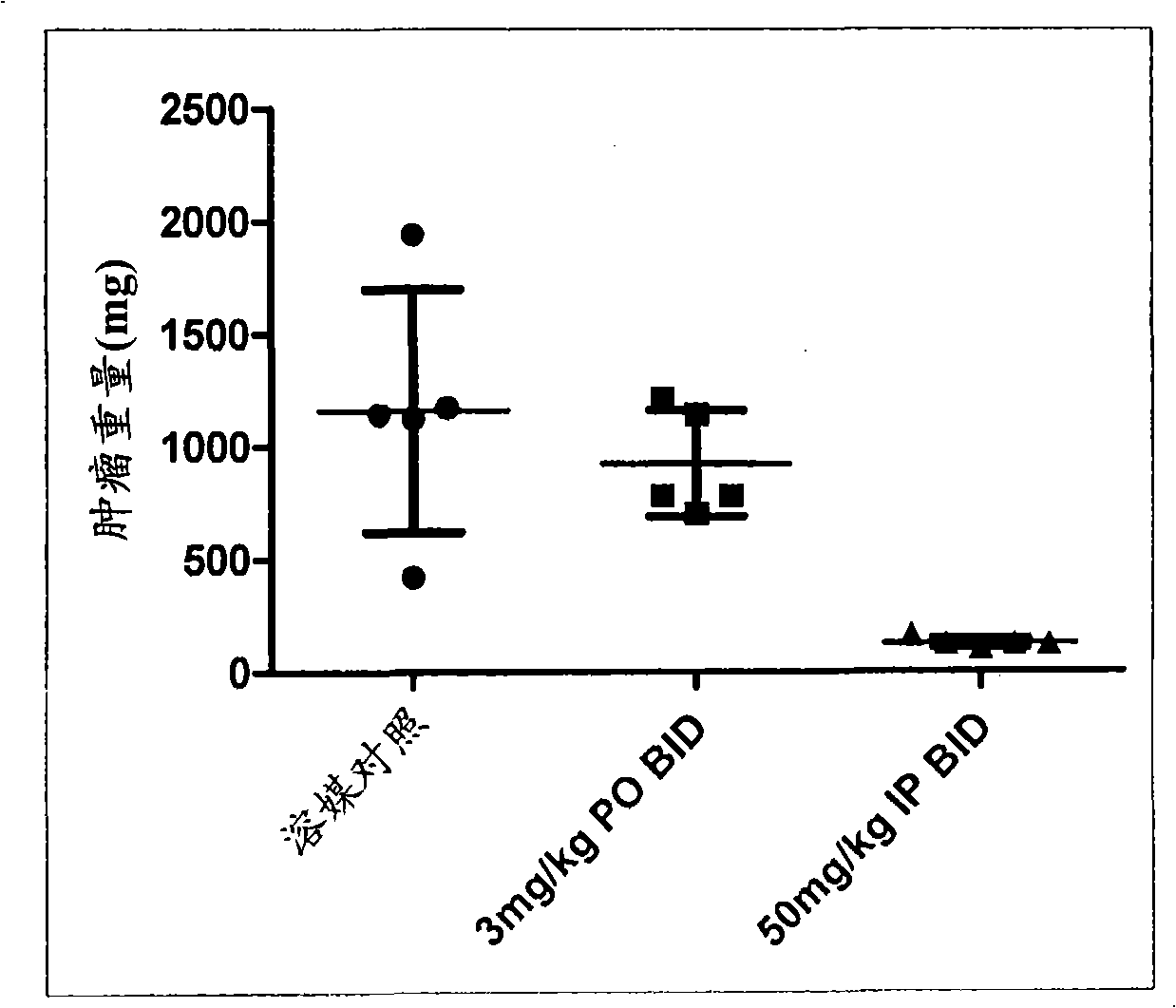
Triazolo-pyridazine protein kinase modulators
An unsubstituted, CH2 technology that can be used in drug combinations, active ingredients of heterocyclic compounds, organic chemistry, etc., and can solve problems such as unclear tractability and lack of kinase activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0374] The following examples are offered to illustrate but not limit the claimed invention. The preparation of embodiments of the invention is described in the following examples. Those of ordinary skill in the art will appreciate that the chemical reactions and synthetic methods provided can be modified to prepare many other compounds of the invention. Where compounds of the invention are not exemplified, those of ordinary skill in the art will recognize that such compounds can be prepared by modifying the synthetic methods set forth herein, as well as by employing synthetic methods known in the art.
[0375] General formula (XII) and (XI) compound, wherein Q, R 1 , X, T and U are described herein and can be prepared according to general reaction scheme 1. Compounds of formula (I) and (II) are either commercially available or prepared from commercially available compounds using standard chemical reactions and transformations known to those skilled in the art.
[0376] Com...
Embodiment 99
[0519] Make 3-[3-(1-quinolin-6-yl-ethyl)-[1,2,4]triazolo[4,3-b]pyridazin-6-yl]-benzonitrile (1.2g ) was dissolved in isopropyl alcohol (10 g / L) and eluted by chiral HPLC on a Chiralpak AS 20 μM (8 cm id x 25 cm L) column with 50% methanol / ethanol (25 °C), 150 g / min, 325 nM To separate the enantiomers, Example 126 (0.56 g, ee > 99.9%) and Example 127 (0.56 g, ee 97%) were recovered. The analytical method was performed on an AS-H 4.6mm ID x 250mm S / NASHSAEE001-409291 column, eluted with 50% methanol / ethanol (25°C), 1 mL / min, 225nM. Compound 99 had a retention time of 4.6 minutes and compound 100 had a retention time of 5.8 minutes. Analytical data are shown in Table 1.
[0520] To a degassed (nitrogen bubbled through for 15 min) mixture of water (1 mL) and 1,4-dioxane (2 mL) was added 6-(6-chloro-[1,2,4]triazolo [4,3-b]pyridazin-3-ylmethyl)-3-(1-methyl-1H-pyrazol-4-yl)-quinoline (117mg, 0.31mmol), vinylboronic acid pinacol Ester (59 μl, 0.35 mmol), potassium carbonate (0.93 ...
Embodiment 103
[0523] Make 3-[3-(1-benzothiazol-6-yl-ethyl)-[1,2,4]triazolo[4,3-b]pyridazin-6-yl]-benzonitrile (5.27 g) Dissolve in dichloromethane (200 mL) and separate the enantiomers on a Berger super critical fluid multigram II system. Separation was carried out on a Chiralcel OD-H (2x15cm) column with 40% methanol / CO 2 (100 bar), 50 mL / min, eluted at 220 nM, recovered Example 103 (2.1 g, retention time 5.31 min, ee>99%) and Example 104 (2.2 g, retention time 6.30, ee>99%).
[0524] Add 52mgs (0.295mmol) of (1H-pyrrolo[2,3-b]pyridin-3-yl)-acetic acid, 1.2eq. (0.354mmol) of O-(7-azabenzotri Azol-1-yl)-N,N,N,N'-tetramethyluronium hexafluoro-phosphate, 1.5eq.(0.443mmol)[6-(1-methyl-1H-pyrazole-4- yl)-pyridazin-3-yl]-hydrazine and 4eq. (1.18mmol) N,N-diisopropylethylamine and dissolved in 4mls dimethyl-formamide. The reaction mixture was stirred at room temperature for 18 hours. After this time, dimethylformamide was removed in vacuo, acetic acid (4mls) was added to the residue and heate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com