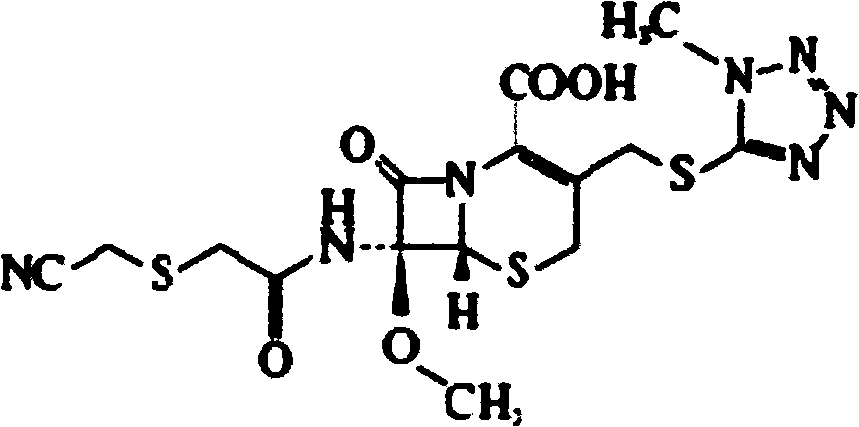
Cefmetazole preparation used for injection and preparation method thereof
A technology of cefmetazole and cefmetazole acid, which is applied to the formulation and preparation of cefmetazole preparations, and can solve the problems of many impurities, low content, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Prescription of Cefmetazole for Injection:
[0036] Cefmetazole acid: 1000g (pure)
[0037] Anhydrous sodium carbonate: 150g
[0038]
[0039] Make 1000 pieces
[0040] Preparation Process:
[0041] Weigh the cefmetazole acid sterile powder and anhydrous sodium carbonate sterile powder according to the prescription amount, mix them evenly, and then pack them into aseptically treated vials, press the stopper, roll the cap, and pass the inspection. After packing.
[0042] The inspection results are shown in Table 3.
[0043] table 3
[0044] project
Embodiment 2
[0046] Prescription of Cefmetazole for Injection:
[0047] Cefmetazole acid: 1000g (pure)
[0048] Anhydrous sodium carbonate: 100g
[0049]
[0050] Make 1000 pieces
[0051] Preparation Process:
[0052] Weigh the cefmetazole acid sterile powder and anhydrous sodium carbonate sterile powder according to the prescription amount, mix them evenly, and then pack them into aseptically treated vials, press the stopper, roll the cap, and pass the inspection. After packing.
[0053] The inspection results are shown in Table 4.
[0054] Table 4
[0055] project
Embodiment 3
[0057] Prescription of Cefmetazole for Injection:
[0058] Cefmetazole acid: 1000g (pure)
[0059] Anhydrous sodium carbonate: 200g
[0060]
[0061] Make 1000 pieces
[0062] Preparation Process:
[0063] Weigh the cefmetazole acid sterile powder and anhydrous sodium carbonate sterile powder according to the prescription amount, mix them evenly, and then pack them into aseptically treated vials, press the stopper, roll the cap, and pass the inspection. After packing.
[0064] The inspection results are shown in Table 5.
[0065] table 5
[0066] project
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com