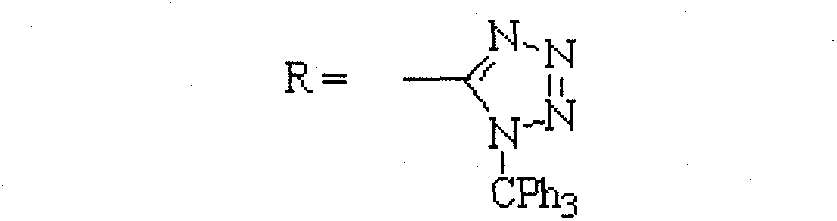Method for purifying sartan side chain compound
A purification method and compound technology, applied in the direction of carboxylic acid nitrile purification/separation, organic chemistry, etc., to achieve the effect of easy recovery and little impact on product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
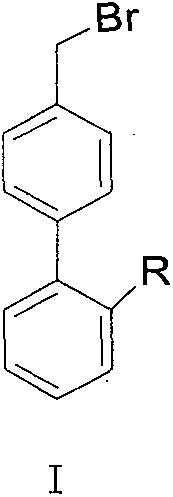
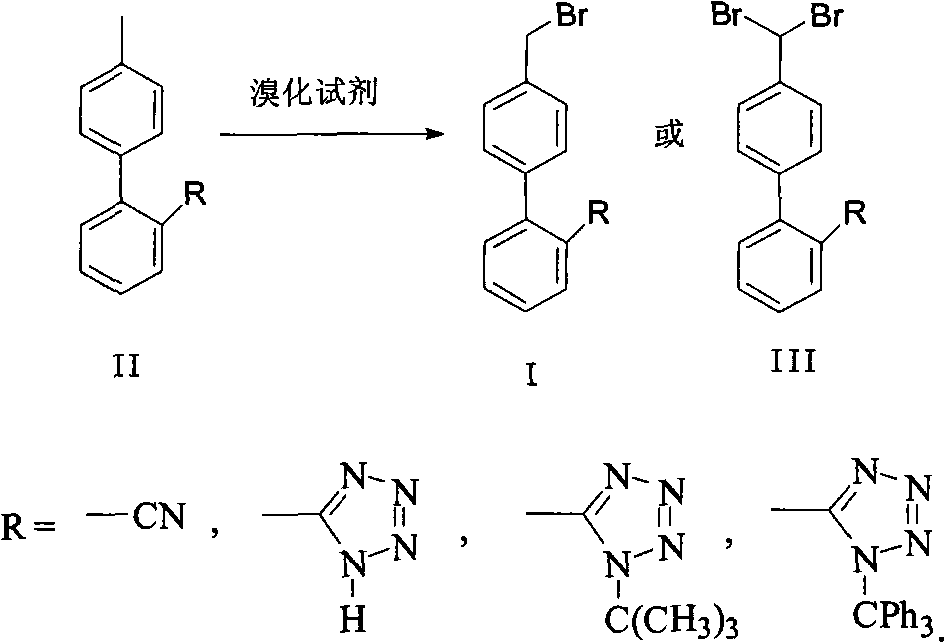
[0021] Example 1: Synthesis of 4'-bromomethyl-2-cyanobiphenyl crude product
[0022] In a 2L three-necked flask, add 96.6g (0.50mol) 4'-methyl-2-cyanobiphenyl (OTBN), 94g bromosuccinimide, 600ml dichloromethane, 2.0g benzoyl peroxide , heated to reflux for 10h, then cooled to room temperature, and filtered, the dichloromethane layer was washed with 400ml of 5% sodium bicarbonate, washed with water, concentrated and evaporated to dryness, and then processed to obtain 136g of 4'-bromomethyl-2-cyanobiphenyl . (HPLC result: Br-OTBN 84.6%, Br 2 -OTBN9.0%, OTBN 6.0%)
Embodiment 2
[0023] Embodiment 2: Purification of 4'-bromomethyl-2-cyanobiphenyl
[0024] The above solid was suspended in 200ml methyl ethyl ketone, heated to 50-55°C, then kept for 2 hours, then gradually cooled to 10-15°C, kept for crystallization for 4 hours, filtered, and 108.8g was obtained after the solid was dried, with a total yield of 80%. Solid purity greater than 98%.
[0025] The above method is used for secondary purification, the purity is greater than 99%, and the secondary purification yield is greater than 93%.
Embodiment 3
[0026] Embodiment 3: Synthesis of crude product of N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl 2-yl)tetrazolium
[0027] In a 500ml three-necked flask, add 47.8g (0.10mol) N-(triphenylmethyl)-5-(4'-methylbiphenyl-2-yl)tetrazolium, 200ml dichloromethane, 19g bromo Succinimide, 1g benzoyl peroxide, warming up to reflux for 5-6 hours, then cooling to room temperature, filtering, washing the dichloromethane layer with 100ml 5% sodium bicarbonate, washing with water, concentrating and evaporating to dryness, and then processing to obtain 55.0 g of crude N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium. (HPLC results: BBTT 82.3%, Br-BBTT 10.4%, BBTT-Br 6.2%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com