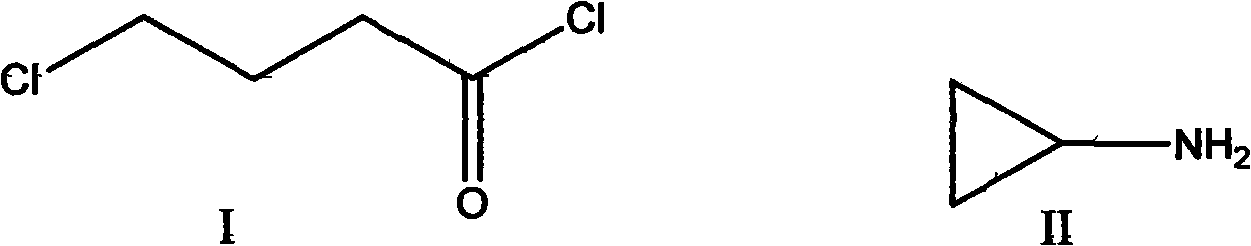
Preparation method of 4-chlorobutyroyl chloride
A technology of chlorobutyryl and butyrolactone, which is applied in the field of preparation of 4-chlorobutyryl chloride, can solve the problems of easy escape of phosgene, high reaction temperature, and no industrial operation, and achieve simple process and high yield High and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
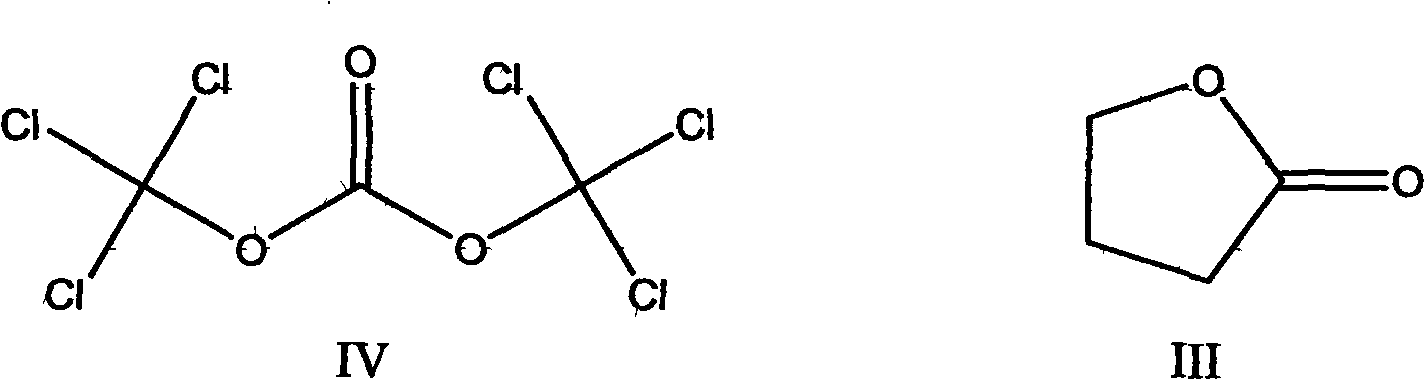
[0036] Add 43g (0.5mol) of γ-butyrolactone and 1.5g of zinc chloride to a 250ml three-neck flask, stir and heat up to 100°C, cool down to room temperature, add 150g (0.505mol) of solid phosgene, heat up to 120°C and keep for 18 hours , and recovered under reduced pressure to obtain 53.1g of 4-chlorobutyryl chloride, with a conversion rate of 75.3%.
Embodiment 2
[0038] Add 43g (0.5mol) of γ-butyrolactone, 1.5g of zinc chloride, and 1 drop of DMF into a 250ml three-neck flask, stir and heat up to 100°C, cool down to room temperature, add 75g (0.253mol) of solid phosgene, and heat up to 100°C After 10 hours of heat preservation, 55.62 g of 4-chlorobutyryl chloride was recovered under reduced pressure, with a conversion rate of 78.9%.
Embodiment 3
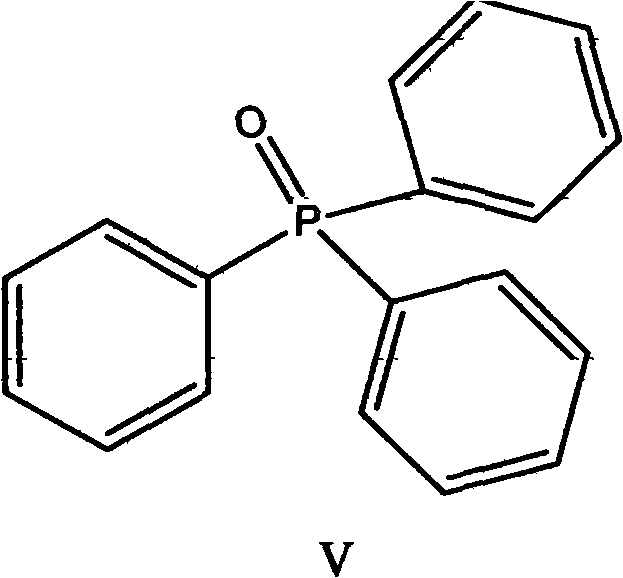
[0040] Add 43g (0.5mol) of γ-butyrolactone, 1.2g of zinc chloride, and 0.02g of triphenylphosphine oxide into a 250ml three-necked flask, stir and heat up to 100°C, cool down to room temperature, and add 75g (0.253mol) of solid phosgene , raised the temperature to 90° C. for 15 hours, and recovered under reduced pressure to obtain 57.88 g of 4-chlorobutyryl chloride, with a conversion rate of 82.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com