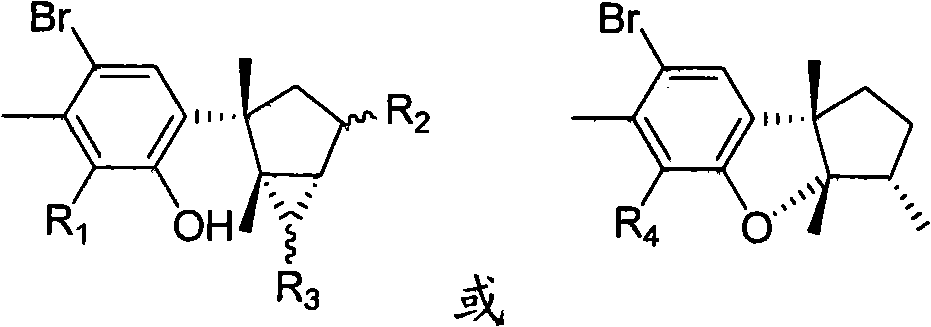
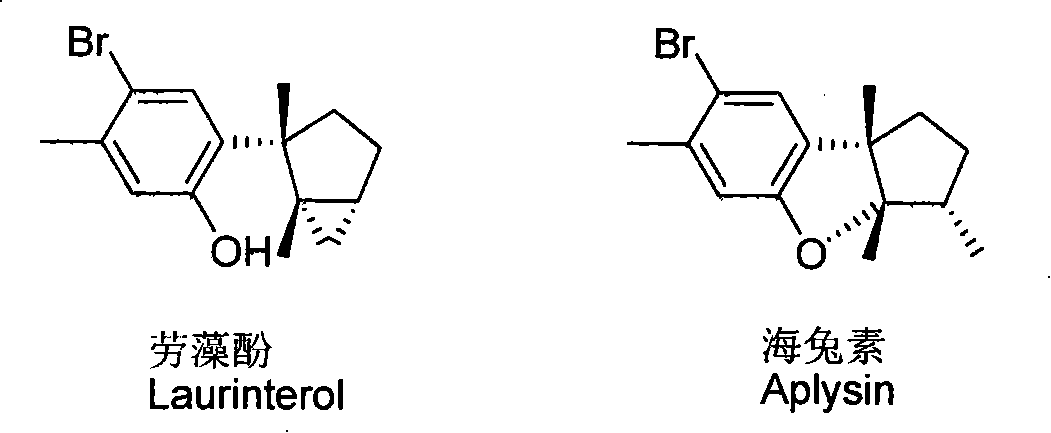
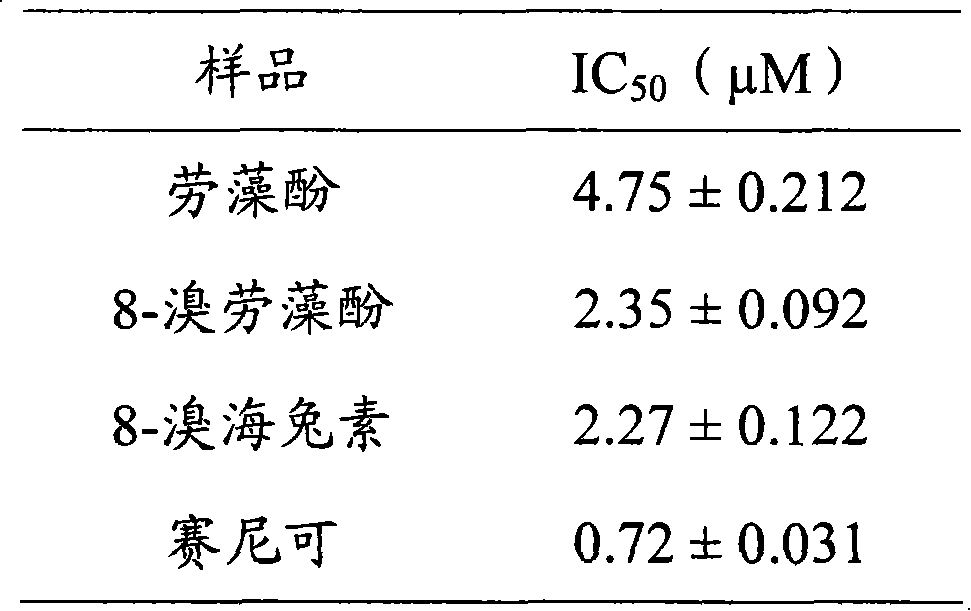
Laurane type sesquiterpene compound or derivative thereof and preparation method and application thereof
A technology of sesquiterpenes and laurane, applied in the directions of digestive system, organic chemistry, drug combination, etc., can solve the problems of uncertain active ingredients and their amounts, difficult to control, insufficient effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of laurane-type sesquiterpenoids Lauzaol and Aplysia
[0028] (1) Extraction: 500g of the dry weight of Suatopina Okamura from East my country Sea, repeated ultrasonic extraction 3 times with 1000ml of acetone, the extracts were combined and concentrated under reduced pressure, the obtained crude extract was suspended in 500ml of aqueous solution, and extracted repeatedly with 500ml of ether. The suspension was collected three times, and the resulting extracts were combined and concentrated under reduced pressure to obtain 36.1 g of ether extract.
[0029] (2) Separation: 36.1g of ether extract was subjected to 200-300 mesh silica gel column chromatography, and petroleum ether / acetone 100:0→98:2→95:5→90:10→80:20→70:30→50 :50 gradient elution, the amount of each gradient is 1000ml; wherein, petroleum ether / acetone 98:2 eluent concentrate obtained 4.65g, through 200-300 mesh silica gel column chromatography, with petroleum ether / diethyl ether 100:0→98 : 2 → 95...
Embodiment 2
[0034] 8-Bromolauralol (R 1 is bromine, R 2 and R 3 at the same time as hydrogen)
[0035] Weigh 12.1 mg of Laualinol sample and dissolve it in 2.0 ml of ethyl acetate solution, add 1.5 times the equivalent of NaBrO 3 Aqueous solution, after mixing well, add 1.5 times equivalent of NaHSO dropwise under stirring 3 Aqueous solution, after reacting at room temperature for 18 hours, was extracted with ethyl acetate to terminate the reaction, and the solvent was recovered from the ethyl acetate extract under reduced pressure to obtain 11.7 mg of 8-bromolauralol.
[0036] The physical and chemical properties and spectral data of 8-bromolauralol are as follows: C 15 h 18 OBr 2 ; 1 H NMR (300MHz, CDCl 3 )δ: 7.70(s, H-11), 5.78(s, -OH), 2.53(s, H 3 -12), 2.23 (dd, J=12.6, 5.1Hz, H-5), 1.64 (dd, J=12.6, 7.2Hz, H-5), 1.94, 1.25 (all m, H 2 -4), 1.40(s, H 3 -13), 1.30 (s, H 3 -14), 1.10(m, H-3), 0.53(m, H 2 -15); EIMS m / z 374, 359, 306 [M] + .
Embodiment 3
[0038] 4,8,15-tribromolauralinol (R 1 , R 2 and R 3 while bromine)
[0039] Weigh 67.5 mg of Laualinol sample and dissolve it in 5.0 ml of chloroform solution, add 1.5 times the equivalent of NaBrO 3 Aqueous solution, after mixing well, add 1.5 times equivalent of NaHSO dropwise under stirring 3 Aqueous solution, after reacting at room temperature for 18 hours, was extracted with ethyl acetate to terminate the reaction, and the solvent was recovered from the ethyl acetate extract under reduced pressure to obtain 26.7 mg of 4,8,15-tribromolauralol.
[0040] The physical and chemical properties and spectral data of 4,8,15-tribromolauralol are as follows: C 15 h 16 OBr 4 ; 1 HNMR (300MHz, CDCl 3 )δ: 7.15(s, H-11), 5.58(s, -OH), 3.58(m, H-4), 3.43(m, H-15), 3.01(m, H-5), 2.69(m , H-5), 2.56(s, H 3 -12), 1.26(s, H 3 -13), 1.04 (s, H 3 -14), 2.54(m, H-3); EIMS m / z 532, 517, 357[M] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com