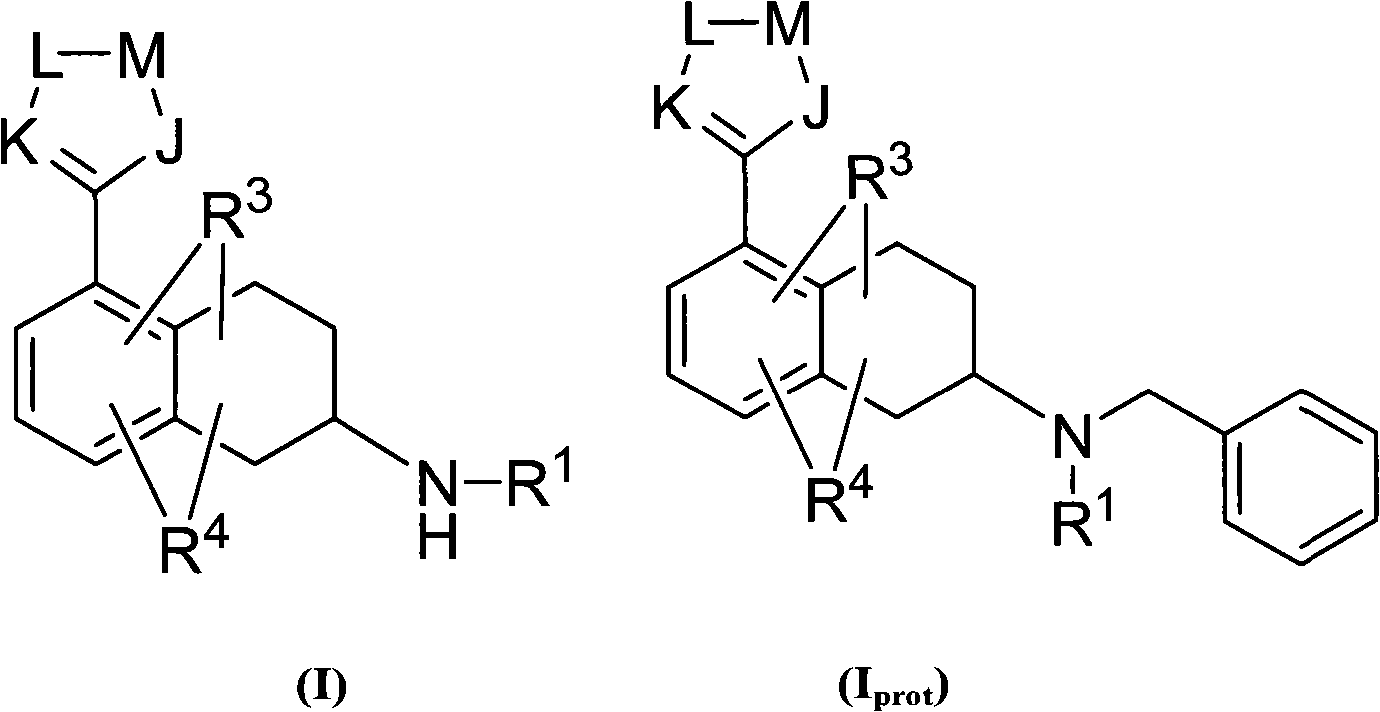
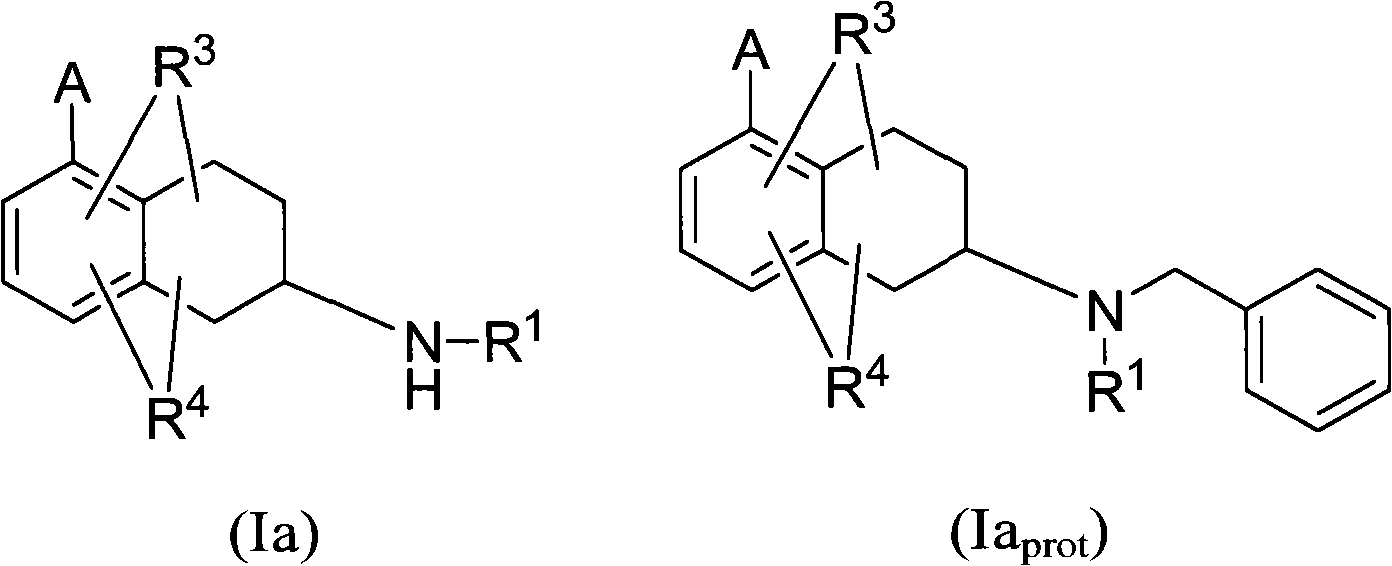
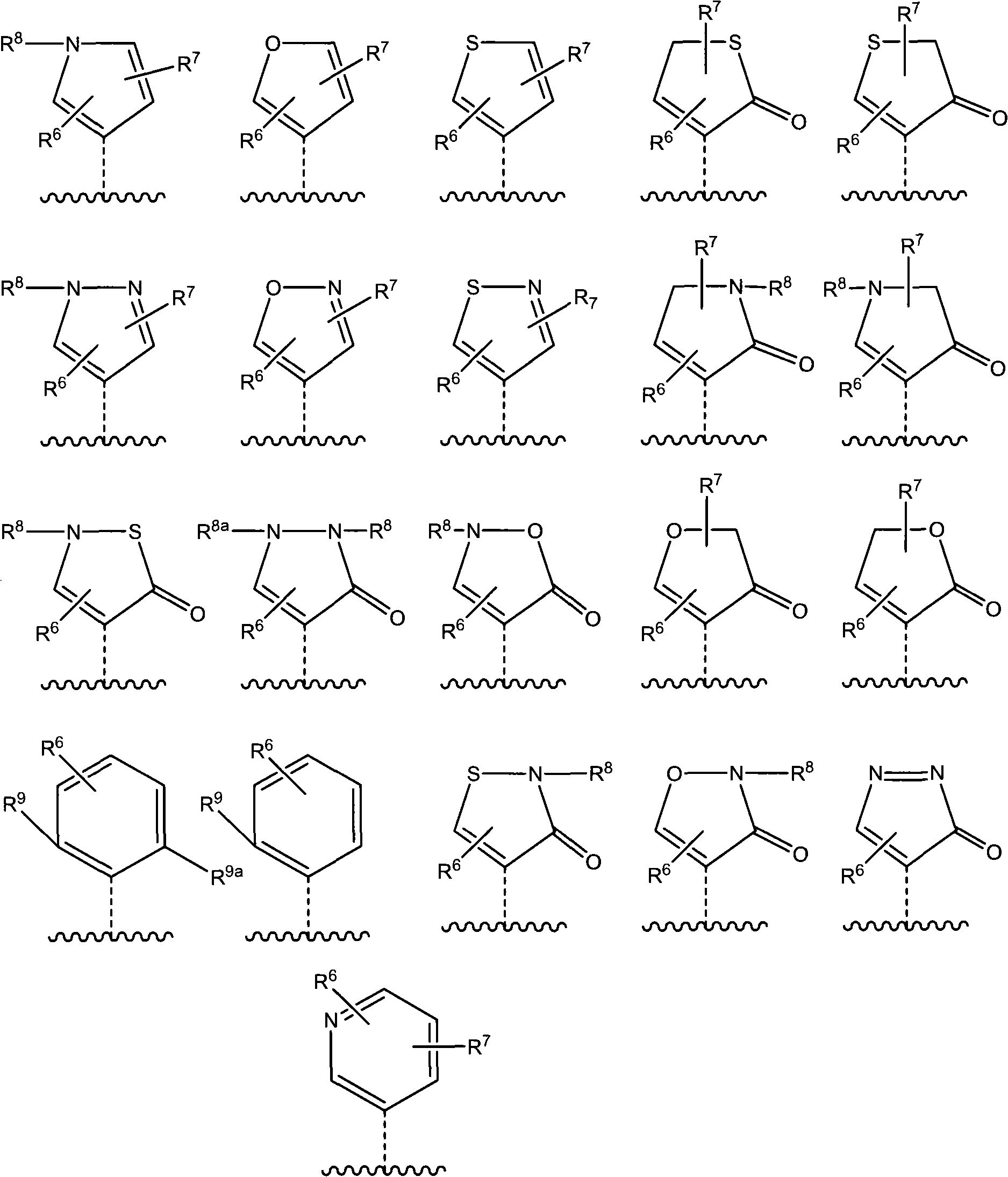
Heterocyclyl-substituted-tetrahydro-naphthalen derivatives as 5-ht7 receptor ligands
A technology of tetralin derivatives and heterocyclic groups, applied in the field of tetralin compounds, can solve problems such as low sequence homology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment G
[0420] Chemical Example G: (2S)-Methyl-[5-(1,3,5-trimethyl-1H-pyrazol-4-yl)-1,2,3,4-tetrahydro-naphthalene-2 -yl]-amine
[0421]
[0422] Methyl-[5-(1,3,5-trimethyl-1H-pyrazol-4-yl)-1,2,3,4-tetrahydro-naphthalen-2-yl]-amine
[0423] Example G was prepared according to the following Scheme 2
[0424]
Embodiment A
[0427] N-Benzyl-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)amine
[0428]
[0429] To dissolve in CH 2 Cl 2 Benzylamine (23 mL, 212.80 mmol) and AcOH (0.97 mL, 17.02 mmol) were added to a solution of 5-methoxy-2-tetralone (30 g, 170.24 mmol) in (250 mL), and the mixture was heated at room temperature Stir for 4h. Then cool to 0°C and add NaB(OAc) within 20min 3 H (0.38 equiv, 13.71 g, 64.69 mmol). After stirring at 0°C for 1 h, NaB(OAc) was added within 30 min 3 H (1.07 equiv, 38.61 g, 182.16 mmol). Join CH 2 Cl 2 (100 mL), the reaction mixture was warmed to room temperature and stirred for 15 h. Then the mixture was cooled to 0 °C, and H was added slowly 2 O (200 mL). By adding saturated NaHCO 3 Aqueous solution (300 mL) was used to adjust the pH of the solution to 8.0, and the mixture was stirred at 0 °C for 15 min. After separation, the aqueous phase was washed with CH 2 Cl 2 (2 x 150 mL) extraction. All organic phases were combined and washed with anh...
Embodiment B
[0432] N-Benzyl-N-[(2S)-5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl]amine
[0433]
[0434] N-Benzyl-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)amine (11.70 g, 43.76 mmol) was dissolved in Et 2 O (350 mL). The reaction mixture was heated to reflux and (S)-(+)-mandelic acid (6.66 g, 43.76 mmol) was added. Join CH 2 Cl 2 (30 mL), the mixture was refluxed for 3 h. The mixture was cooled at room temperature and stirred for 16h. The obtained solid was filtered and washed with Et 2 Washing with O (3 x 20 mL) and drying afforded 6.80 g of the diastereomeric salt as a white solid. Evaluation of the ee value in the compound (6S)-6-(dimethylamino)-5,6,7,8-tetralin-1-ol (chiral column chiralCel OD-H, 5 μm, 4.6×250mm; flow rate : 1 ml / min; mobile phase: MeOH:EtOH (1:1) / hexane 10:90). Diastereoisomeric salts from Et 2 Recrystallization in O improves ee. The salt was suspended in AcOEt (100 mL), and K 2 CO 3 Aqueous solution (20%, 80 mL). The mixture was stirred at room ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com