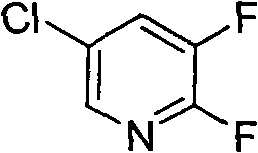
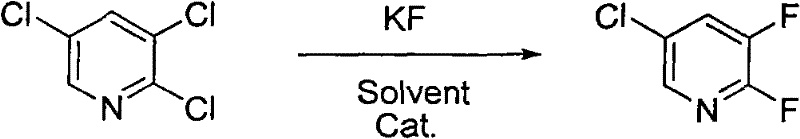
Synthesis method of 2,3-difluoro-5-chloropyridine
A synthesis method, the technology of chloropyridine, applied in 2 fields, can solve the problem of high price, achieve the effect of simple operation and low solution yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 2,3,5-Trichloropyridine (18.2g, 0.1mol), potassium fluoride (13.9g, 0.24mol, pre-vacuum-dried at 140°C for 12 hours) were dissolved in 50mL sulfolane, under nitrogen protection, heated to 120°C, Added 1.2 g of tetraphenylphosphine bromide, maintained at 180°C for 5 hours, and then maintained at 200°C for 12 hours. According to gas phase analysis, the yield of 2,3-difluoro-5-chloropyridine was 39.1%, and the conversion rate of 2,3,5-trichloropyridine was 99%.
Embodiment 2
[0026] 2,3,5-Trichloropyridine (18.2 g, 0.1 mol), potassium fluoride (17.4 g, 0.3 mol, pre-vacuum-dried at 140°C for 12 hours) were dissolved in 100 mL of N-methylpyrrolidone, protected by argon. Heated to 120°C, added 2.0 g of tetrabutylphosphine bromide, maintained at 185°C for 7 hours, and then maintained at 205°C for 15 hours. According to gas phase analysis, the yield of 2,3-difluoro-5-chloropyridine was 42%, and the conversion rate of 2,3,5-trichloropyridine was 100%.
Embodiment 3
[0028] 2,3,5-Trichloropyridine (18.2g, 0.1mol), potassium fluoride (23.1g, 0.4mol, pre-vacuum-dried at 140°C for 12 hours) were dissolved in 100mL N-methylpyrrolidone, protected by argon, Heated to 120°C, added 1.0g of tetrabutylphosphine bromide, maintained at 190°C for 10 hours, and then maintained at 205°C for 10 hours, the yield of 2,3-difluoro-5-chloropyridine was 40%, 2 , What is the conversion rate of 3,5-trichloropyridine 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com