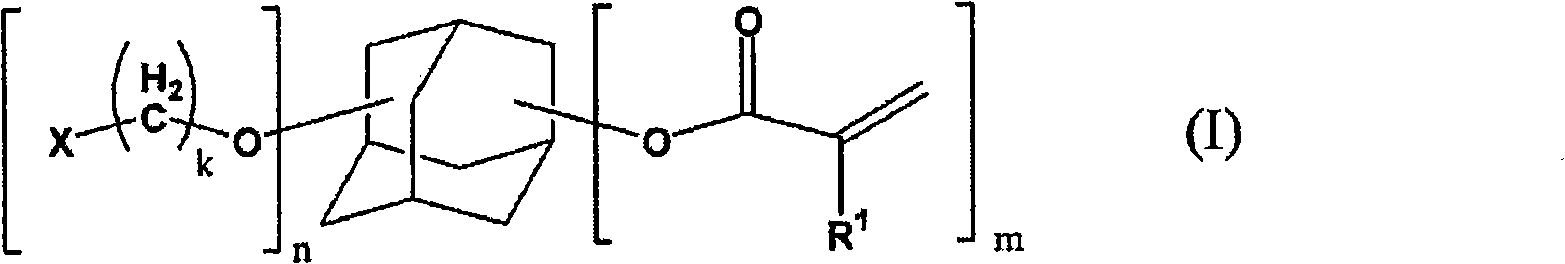
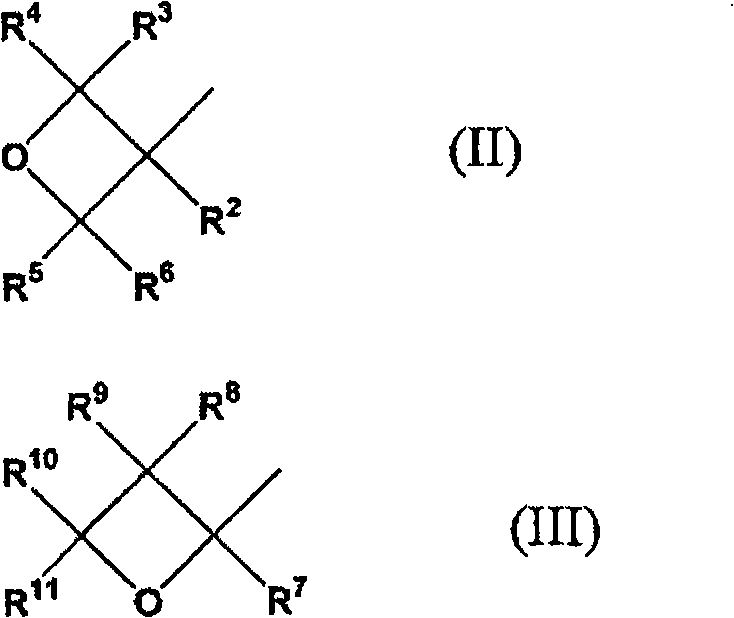
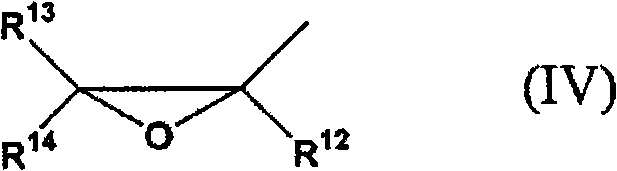
Adamantane derivative, resin composition using the same, and resin cured product
A technology of adamantane and derivatives, applied in the field of adamantane derivatives and its preparation, can solve the problems that have not yet been obtained, and achieve the effect of excellent dielectric constant and excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Synthesis of 3-[(3-ethyloxetan-3-yl)methoxy]-1-adamantyl methacrylate
[0135] Add 50.4g (0.160mol) 3-methylsulfonyloxy-1-adamantyl methacrylate, 27.2g (0.344mol) Pyridine, 0.01 g of methoquinone, and 200 g of (3-ethyloxetan-3-yl)methanol (manufactured by Ube Industries, trade name: Ethanacol EHO) were used for nitrogen substitution. Then the temperature was raised to 120° C., and heated and stirred for 4 hours. The reaction solution was cooled, extracted with toluene, and washed with saturated brine. The solvent was distilled off under reduced pressure to obtain 42.7 g (yield 74%) of the target product. given below 1 H-NMR, 13 Respective data of C-NMR and GC-MS.
[0136]
[0137] 1 H-NMR (500MHz): 0.85(3H), 1.55(2H), 1.67-1.78(4H), 1.89(2H), 2.06-2.20(6H), 2.33(2H), 3.53(2H), 4.35-4.40( 4H), 5.48(1H, a2), 6.00(1Ha1)
[0138] 13 C-NMR(125MHz): 8.25(q), 18.39(c), 26.64(p), 31.06(g), 35.26(h), 40.35(f or j), 40.51(f or j), 43.18(m) , 45.32(i), 62.95(k), 73.85...
Embodiment 2
[0141] Synthesis of 3-[(3-ethyloxetan-3-yl)methoxy]-1-adamantyl acrylate
[0142] Add 50.4g (0.167mol) 3-methylsulfonyloxy-1-adamantyl acrylate, 27.2g (0.344mol) pyridine, Nitrogen substitution was carried out with 0.01 g of methylbenzoquinone and 200 g of (3-ethyloxetan-3-yl)methanol (manufactured by Ube Industries, trade name: Ethanacol EHO). Then the temperature was raised to 120° C., and heated and stirred for 4 hours. The reaction solution was cooled, extracted with toluene, and washed with saturated brine. The solvent was distilled off under reduced pressure to obtain 38 g (yield 71.2%) of the target product. given below 1 H-NMR and 13 Each data of C-NMR.
[0143]
[0144] 1 H-NMR (500MHz): 0.85(3H), 1.55(2H), 1.67-1.78(4H), 1.89(2H), 2.06-2.20(6H), 2.33(2H), 3.53(2H), 4.35-4.40( 4H), 5.69 (dd, J=1.6, 10.7Hz, 1H, a2), 5.97 (dd, J=10.7, 17.6Hz, 1H, b), 6.24 (dd, J=1.6, 17.6Hz, 1H, a1)
[0145] 13 C-NMR (125MHz): 8.25(p), 26.64(o), 30.96(f), 35.04(g), 40.20(e o...
Embodiment 3
[0147] Synthesis of 3-[(3-oxiran-2-yl)methoxy]-1-adamantyl methacrylate
[0148] Add 50.4g (0.160mol) 3-methylsulfonyloxy-1-adamantyl methacrylate, 27.2g (0.344mol) Pyridine, 0.01 g of methylbenzoquinone, and 200 g of 2-chloro-1,3-propanediol were used for nitrogen substitution. Then the temperature was raised to 80° C., and heated and stirred for 2 hours. The reaction solution was cooled, then extracted with 500 ml of toluene, and washed twice with 500 ml of saturated brine. 20 g of sodium hydroxide was added to the solution after washing, and after heating and stirring at 110° C. for 2 hours, the solvent was cooled. Then, it was washed twice with 300 mL of saturated brine, and the solvent was distilled off under reduced pressure to obtain 40 g (yield 82%) of the target product. given below 1 H-NMR and 13 Each data of C-NMR.
[0149]
[0150] 1 II-NMR (500MIIz): 1.67-1.78(4II), 1.89(2II), 2.06-2.20(6II), 2.33(2H), 2.38(1H), 2.63(1H), 2.86(1H), 3.53(2H) , 5.48(1H, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com