Undoped red organic electroluminescent material and preparation method and applications thereof
A reaction and compound technology, applied in the field of organic electroluminescence, can solve the problems of energy matching, phase separation and carrier transport imbalance, increase of non-radiative deactivation process, weakening of fluorescence quantum efficiency, etc. Transmission capability, reduction of dipole-dipole interactions, effects of cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
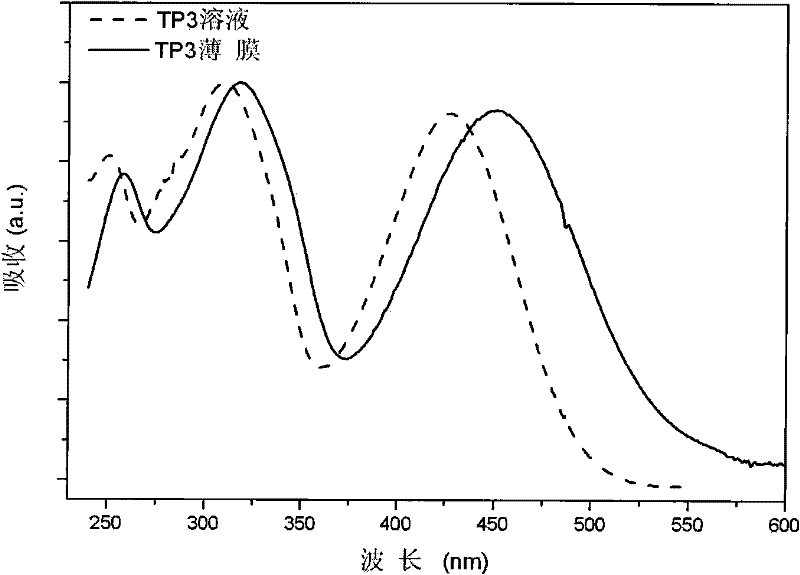
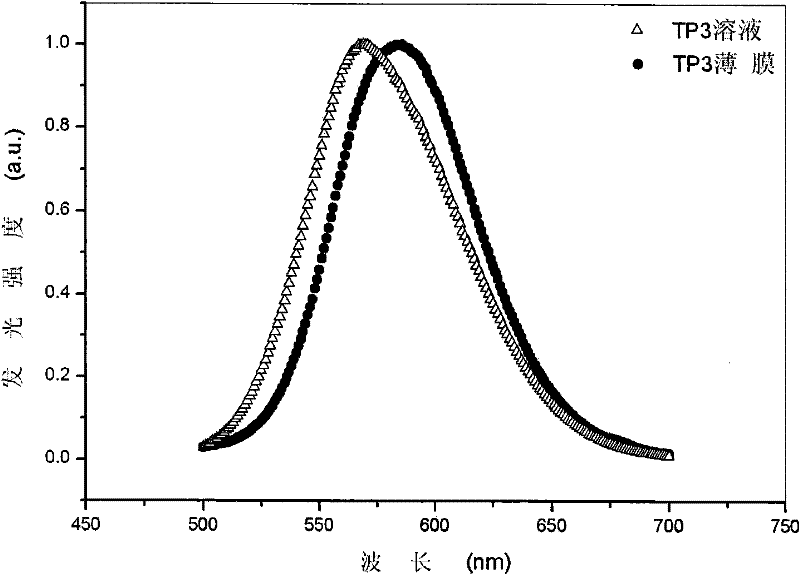
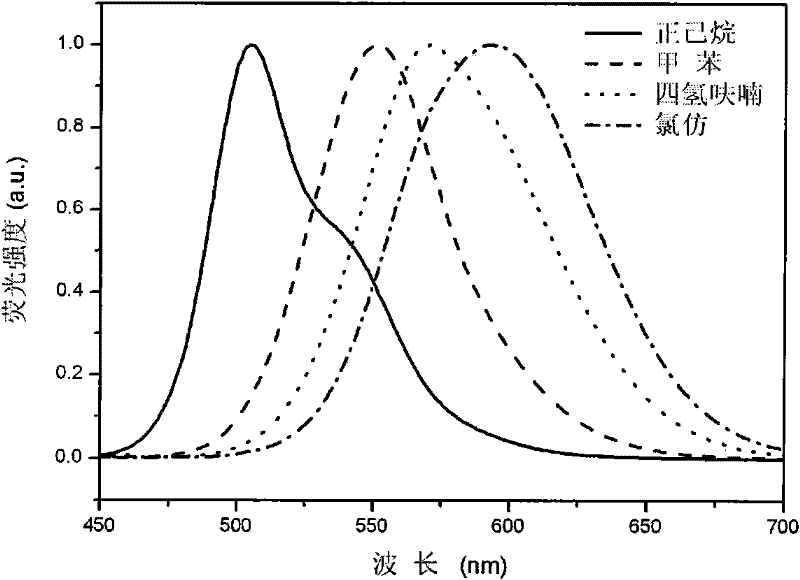
[0050] Example 1 Preparation of non-doped red organic electroluminescent material TP3 and its properties
[0051] 1. Preparation of 2,4,6-trimethyl-1,3,5-s-triazine (compound 1)
[0052] Mix 24.6g of acetonitrile (0.6mol) and 27.6g of absolute ethanol (0.2mol) in a three-necked flask, and feed hydrogen chloride gas at room temperature until 0.6mol of hydrogen chloride is absorbed, that is, the weight of the reaction solution increases by 14.3g. Stir at room temperature for 16 hours. After the reaction was complete, the crude product was filtered off and dried. After drying, it was added to a vigorously stirred mixture of 50 mL of dichloromethane, 125 mL of water and potassium carbonate (0.6 mol). After stirring for 10 minutes the organic layer was separated. The aqueous solution was extracted twice with 50 mL of dichloromethane (25 mL each time). The organic phases were combined and dried overnight with anhydrous potassium carbonate in the refrigerator. Most of the solven...
Embodiment 2
[0072] Example 2 Preparation of single-layer non-doped red electroluminescent device
[0073] In this example, a non-doped red electroluminescent device was prepared according to the following method:
[0074] a) Cleaning ITO (indium tin oxide) glass: ultrasonically clean the ITO glass with deionized water, acetone, and ethanol for 15 minutes each, and then treat it in a plasma cleaner for 2 minutes;
[0075] b) Spin-coat PEDOT:PSS on anodic ITO glass as a hole injection layer at 4000 rpm, anneal at 200°C for 5 minutes in air, and then anneal at 200°C for 15 minutes in nitrogen. The thickness is 28nm;
[0076] c) Spin-coat the luminescent layer TP3 on the PEDOT:PSS layer, the concentration of TP3 is 12mg / mL, the rotating speed is 2000 rpm, and the thickness is 80nm;
[0077] d) Vacuum evaporation of LiF on the light-emitting layer TP3, the rate Thickness 0.5nm;
[0078] e) A cathode Al is vacuum-evaporated on the LiF with a thickness of 120 nm.
[0079] The structure of ...
Embodiment 3
[0080] Example 3 Preparation of double-layer non-doped red electroluminescent device
[0081] In this example, a non-doped red electroluminescent device was prepared according to the following method:
[0082] a) cleaning ITO glass: ultrasonically clean the ITO glass with deionized water, acetone, and ethanol for 15 minutes respectively, and then process it in a plasma cleaner for 2 minutes;
[0083] b) Spin-coat PEDOT:PSS on anodic ITO glass as a hole injection layer at 4000 rpm, anneal at 200°C for 5 minutes in air, and then anneal at 200°C for 15 minutes in nitrogen. The thickness is 28nm;
[0084] c) Spin-coat the luminescent layer TP3 on the PEDOT:PSS layer, the concentration of TP3 is 9 mg / mL, the rotation speed is 2000 rpm, and the thickness is 40 nm. ;
[0085] d) Vacuum-evaporated electron transport layer Alq on the light-emitting layer TP3 3 (8-hydroxyquinoline aluminum), rate Thickness 30nm;
[0086] e) In the electron transport layer Alq 3 Vacuum-evaporated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap