Methods for preserving renal function using xanthine oxidoreductase inhibitors
A kidney function and compound technology, applied in the field of xanthine oxidoreductase inhibitory compounds or their salts, can solve problems such as incomplete understanding of renal function effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Before study enrollment, information was collected prospectively in a subgroup of 18 human subjects with a history of nephrolithiasis, based on subject reports. In the 4-week double-blind phase 2 study, subjects were randomly assigned to one of 4 treatment groups: (1) febuxostat 40 mg / day, (2) febuxostat 80 mg / day, (3) Febuxostat 120 mg / day, or (4) placebo.
[0116] Subjects who completed the double-blind study entered the open-label long-term study and began treatment with 80 mg febuxostat / day. The dose of febuxostat can be gradually adjusted to 40 mg or 120 mg febuxostat / day within the initial 6 months based on the subject's serum urate level and the occurrence of adverse events.
[0117] In the study subgroup, a causal analysis of nephrolithiasis was performed in study subjects (n=13) who had received febuxostat for > 30 months. In the event of kidney stone formation, all these stones are analyzed for their mineral content.
[0118] The following were the criteria...
Embodiment 2
[0137] Initially 6-week-old B6C3F1 species / strain mice were dosed by oral gavage with febuxostat suspended in 0.5% methylcellulose. The daily dose administered was 0 mg (ie the control group), 3 mg, 12 mg, 24 mg or 48 mg. Histopathological examination of the kidneys was performed 13 weeks after dosing for vesicular degeneration of the renal proximal tubules, a change known to occur naturally in rodents. The results are shown in Table 5.
[0138] table 5
[0139]
[0140] M = male; F = female
[0141] * p≤0.05 (Dunnett's nonparametric multiple comparison test)
[0142] ** p≤0.01 (Dunnett's nonparametric multiple comparison test)
[0143] Example 2 shows that administration of febuxostat reduces vesicular degeneration of renal proximal tubules in a statistically significant manner in the male animals studied.
Embodiment 3
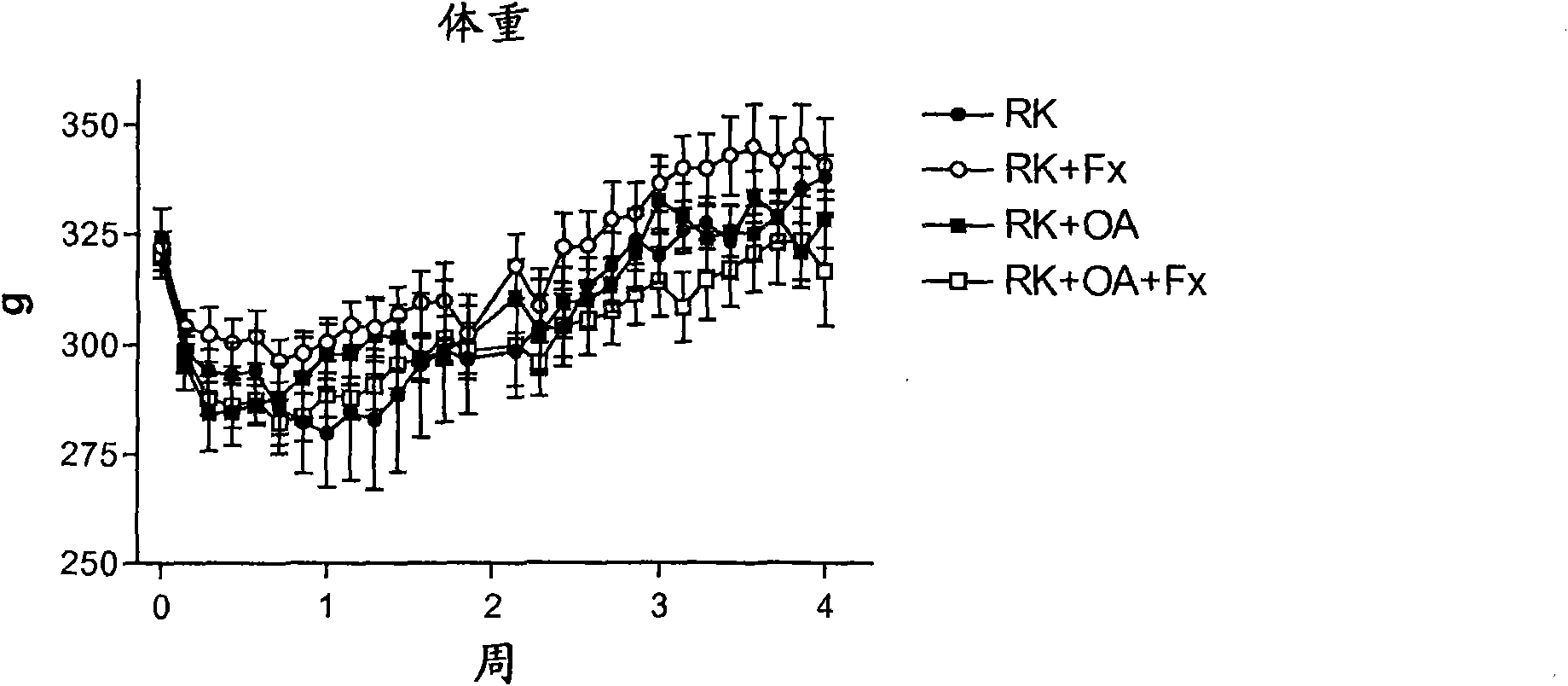
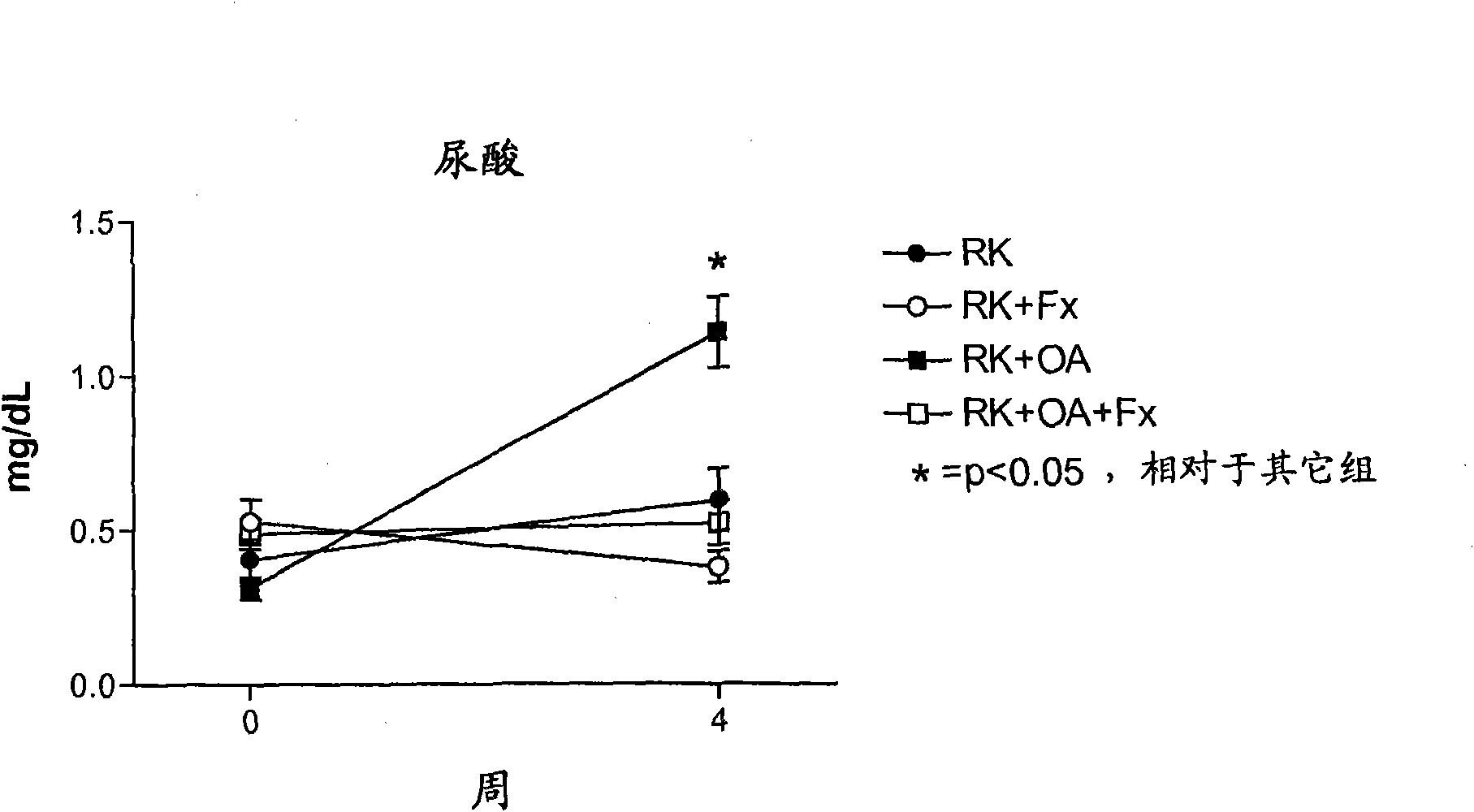
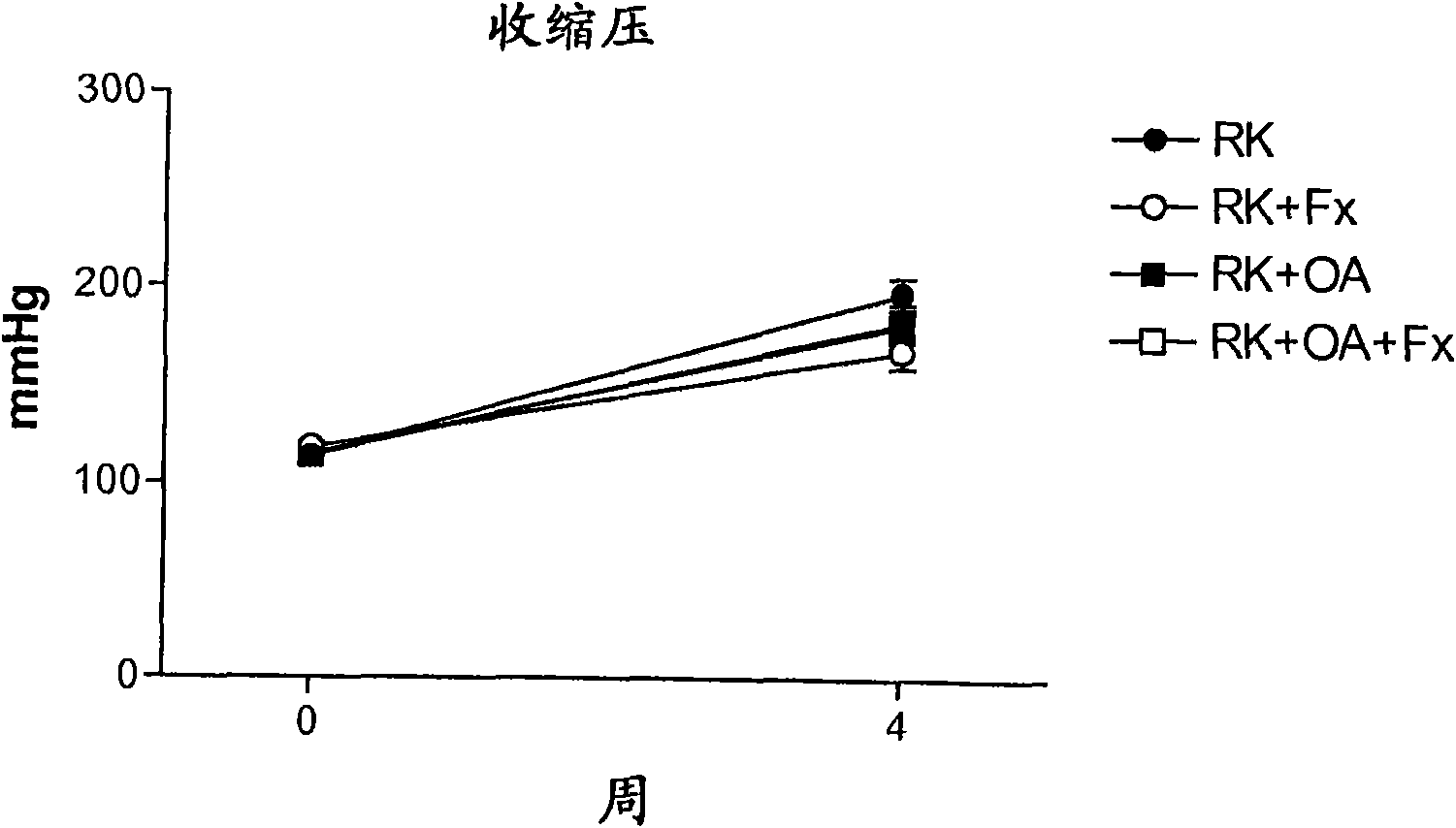
[0145] Rats with remnant kidneys (RK) were generated using male Wistar rats (295-340 g) as follows. Under light anesthesia with ether, a 5 / 6 nephrectomy was performed by removing the right kidney and selectively ligation of 2-3 branches of the left renal artery. The rats were then assigned one of four treatment groups: Group 1, RK control rats (n=7); Group 2, RK+Febuxostat (Fx) rats (n=8); Group 3, RK+oxonic acid (OA) rats (n=6); and Group 4, RK+OA+Fx (n=10). On the day after 5 / 6 nephrectomy, oxonic acid (OA) (Sigma-Aldrich, St Louis Mo., USA) was intragastrically administered at 750 mg / kg body weight per day. Febuxostat was administered at 30 mg / L (3-4 mg / kg / day) in drinking water starting immediately after surgery, while corresponding controls received only drinking water (addition of 3.5 mg / L NaCl, and water containing Fx maintain an equal salt concentration).
[0146] All groups were treated for 4 weeks. Body weight (beginning just before surgery) and food and water in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com