Method for simultaneously screening superoxide anion remover and xanthine oxidase inhibitor
A technology of xanthine oxidase and superoxide anion, which is applied in material separation, instruments, measuring devices, etc., can solve the problems of influence of result accuracy, poor parallelism and reproducibility, poor selectivity, etc., to avoid false positives and false positives. Negative result, good stability, good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
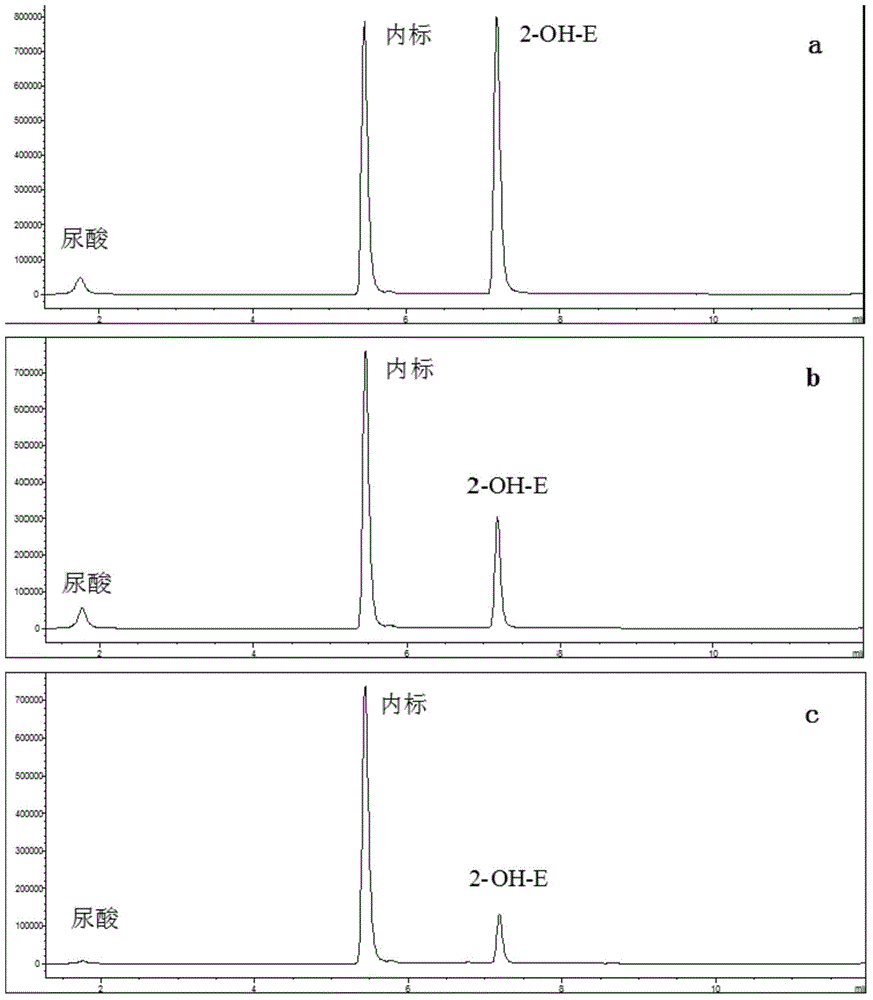
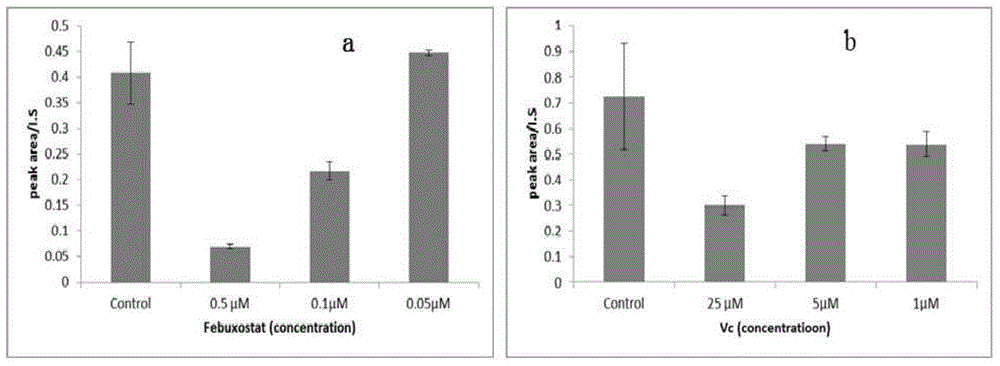
[0058] In order to examine the reliability of the method in this embodiment, Vc is used as O 2 - · As the positive drug of scavenger, febuxostat is used as the positive drug of XOD inhibitor, and the established method is verified.
[0059] (1) Buffer preparation: Precisely weigh KH 2 PO 4 0.0956g, K 2 HPO 4 ·3H 2 O 0.6946g, EDTA 1.862mg, and dilute to 50mL with ultrapure water to obtain a phosphate buffer (PB) containing 75mmol / L phosphate ions and having a pH value of 7.4.
[0060] (2) Preparation of substrate: Dissolving and preparing 40 mmol / L xanthine standard solution with 0.1 mol / L NaOH solution. Dilute with PB before use to obtain a 400 μmol / L substrate solution (the substrate solution needs to be freshly prepared every day).
[0061] (3) Enzyme preparation: 5 U / mL xanthine oxidase stock solution was prepared with phosphate buffered saline and stored in a -80°C refrigerator. Take it out before use, prepare 0.08U / mL working enzyme solution with phosphate-buffere...
Embodiment 2
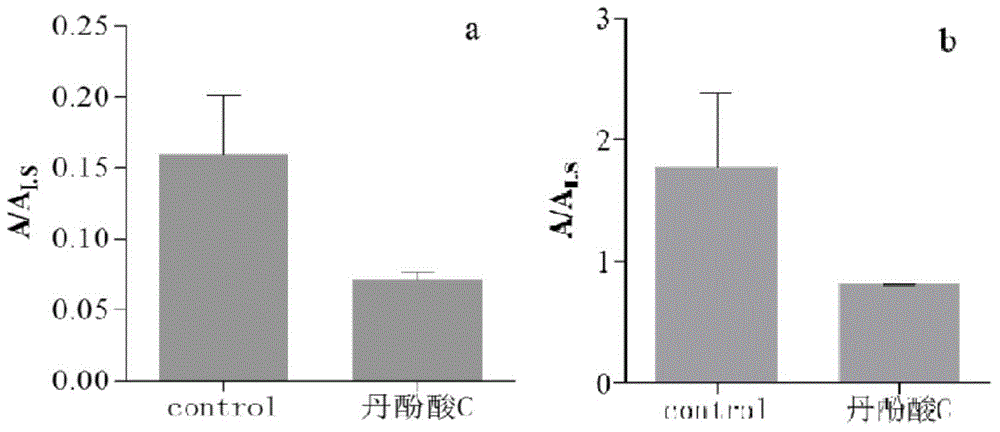
[0087] The xanthine oxidase inhibitor described in this example is salvianolic acid C.
[0088] The sample is as follows: the total reaction volume is 200 μL, the salvianolic acid C with a final concentration of 100 μmol / L and the xanthine oxidase with a final concentration of 0.02 U / mL are co-incubated at 37°C for 2 minutes, and the final concentration of 100 μmol / L is added Xanthine and a fluorescent probe solution with a final concentration of 50 μmol / L were used to start the reaction. After incubation at 37°C for 5 minutes, the reaction was terminated by adding acetonitrile, and the internal standard galantamine with a final concentration of 6 μmol / L was added. After vortexing, centrifuge at 13,000 g After 10 minutes, the supernatant was taken as a sample for determination by ultra-high performance liquid chromatography-mass spectrometry.
[0089] The rest are the same as in Example 1; detect and calculate the 100 μmol / L salvianolic acid C standard substance to xanthine ox...
Embodiment 3
[0091] The xanthine oxidase inhibitor described in this embodiment is quercetin.
[0092] The sample is as follows: the total reaction volume is 200 μL, quercetin with a final concentration of 100 μmol / L is co-incubated with xanthine oxidase with a final concentration of 0.02 U / mL at 37°C for 2 minutes, and a substrate with a final concentration of 100 μmol / L is added Xanthine and fluorescent probe solution with a final concentration of 50 μmol / L start the reaction, incubate at 37°C for 5 minutes, add acetonitrile to terminate the reaction, add internal standard galantamine with a final concentration of 6 μmol / L, vortex and centrifuge at 13,000 g for 10 minutes , take the supernatant as a sample for ultra-high performance liquid chromatography-mass spectrometry determination.
[0093] The rest are the same as in Example 1; detect and calculate the 100 μmol / L quercetin standard substance to xanthine oxidase inhibitory rate 67.72% according to the detection steps of Example 1, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com