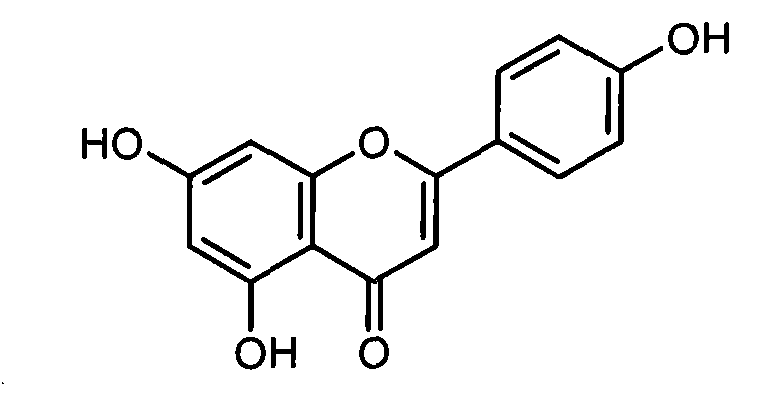
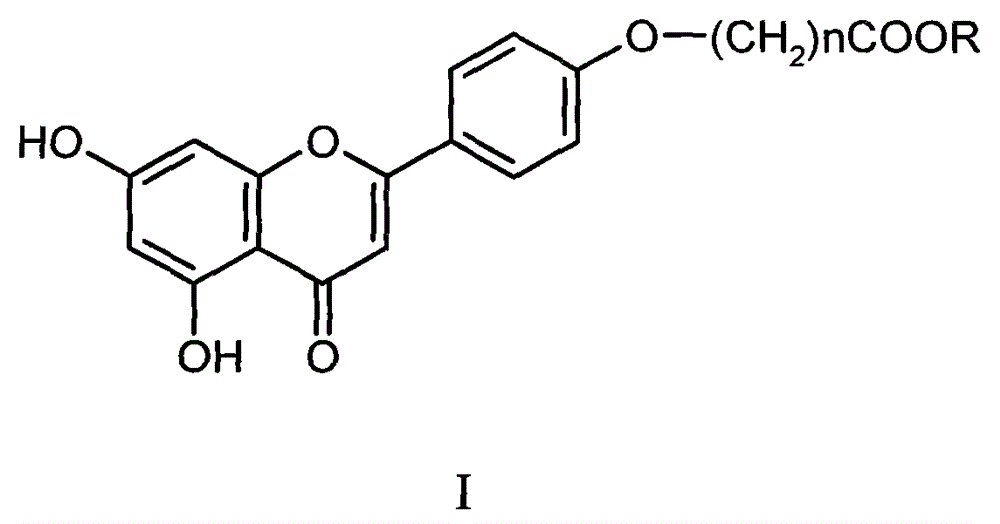
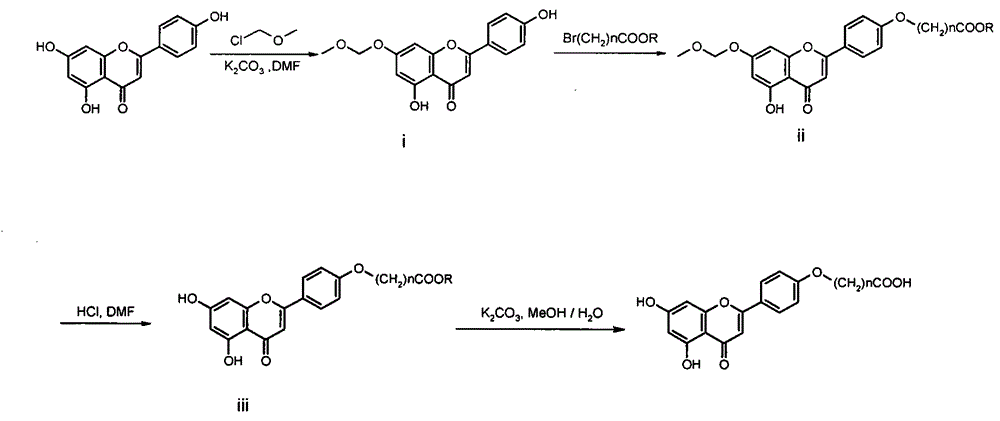
New xanthine oxidase inhibitor
A flavonoid and pharmaceutical technology is applied to the application of a pharmaceutical composition in the preparation of uric acid-lowering drugs. It can solve the problem that the selectivity of the pharmacological action of apigenin needs to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 5, the synthesis of 7-dihydroxy-4'-(1-carboxymethoxy) flavone (I-1)
[0016] 1.1 Preparation of 7-methoxymethyleneoxy-5,4'-dihydroxyflavone (i)
[0017] Add 50 mL of anhydrous DMF to a 250 mL three-necked flask, and add 10 g (37 mmol) of apigenin and 4.1 g (29.6 mmol) of anhydrous potassium carbonate under stirring in an ice bath. Under the protection of argon, 2.97 g (37 mmol) of chloromethyl methyl ether was slowly added dropwise to the reaction system. After the dropwise addition, the ice bath was removed, and the reaction was carried out at room temperature for 12 hours. The reaction was monitored by TLC. After the reaction, potassium carbonate was removed by filtration, 50 mL of water was added, and the pH was adjusted to neutral with dilute hydrochloric acid. Add ethyl acetate and extract (100 mL×3). The ethyl acetate layer was washed successively with saturated sodium chloride solution and water, and dried over anhydrous sodium sulfate. Anhydrous so...
Embodiment 2
[0024] Embodiment 2 5, the synthesis of 7-dihydroxy-4'-(3-carboxypropoxyl) flavone (I-2)
[0025] Referring to Example 1.2, replace ethyl 2-bromoacetate with ethyl 4-bromobutyrate, and react with compound i to obtain 7-methoxymethyleneoxy-5-hydroxyl-4'-(3-ethoxycarbonyl Propoxy) flavone (ii-2), yield: 43%.
[0026] Referring to Example 1.3, ii-2 was demethoxymethylene with 3N hydrochloric acid to obtain 5,7-dihydroxy-4'-(3-ethoxycarbonylpropoxy)flavone (iii-2), producing rate of 85%.
[0027] Referring to Example 1.4, iii-2 was hydrolyzed with potassium carbonate base to obtain 0.18 g of a yellow solid of I-2, namely 5,7-dihydroxy-4'-(3-carboxypropoxy)flavone, with a yield of 90%. Melting point: 232-233°C, 1 H NMR (400MHz, DMSO-d 6 ): δ = 1.95-1.99 (2H, m), 2.41 (2H, t), 4.10 (2H, t), 6.20 (1H, d, J = 2.24Hz), 6.51 (1H, d, J = 2.24Hz) , 6.88(1H, s), 7.11(2H, d, J=8.96Hz), 8.03(2H, d, J=8.96Hz), 10.87(1H, s), 12.19(1H, s), 12.93ppm(1H , s); HRMS-ESI(MeOH): m / z[M-H] - C ...
Embodiment 3
[0028] Example 3 5,7-dihydroxy-4,-(4-carboxybutoxy)flavone (I-3) synthesis
[0029] Referring to Example 1.2, replace ethyl 2-bromoacetate with ethyl 5-bromovalerate, and react with compound i to obtain 7-methoxymethyleneoxy-5-hydroxyl-4'-(4-ethoxycarbonyl Butoxy)flavone (ii-3), yield: 46%.
[0030] Referring to Example 1.3, ii-3 was demethoxymethyleneized with 3N hydrochloric acid to obtain 5,7-dihydroxy-4'-(4-ethoxycarbonylbutoxy)flavone (iii-3), producing The rate is 95%.
[0031] Referring to Example 1.4, iii-3 was hydrolyzed with potassium carbonate base to obtain 0.18 g of a yellow solid of I-3, namely 5,7-dihydroxy-4'-(4-carboxybutoxy)flavone, with a yield of 90%. Point: 202-204°C, 1 H NMR (400MHz, DMSO-d 6): δ=1.64-1.68 (2H, m), 1.74-1.78 (2H, m), 2.30 (2H, t), 4.09 (2H, t), 6.20 (1H, d, J=2.24Hz), 6.51 ( 1H, d, J = 2.24Hz), 6.88 (1H, s), 7.11 (2H, d, J = 8.96Hz), 8.03 (2H, d, J = 8.96Hz), 10.87 (1H, s), 12.00 ( 1H, s), 12.94ppm (1H, s); HRMS-ESI (MeOH): m / z [M-H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com