New application of catechin compound and gallic acid combination to preparation of hyperuricemia treatment medicine
A technology of hyperuricemia and catechins, applied in the field of pharmaceutical compositions that can treat hyperuricemia and gout, can solve the problems of stimulating acute attacks of gout, large toxic and side effects of drugs, and weak inhibitory effect. Toxic and side effects, lower serum uric acid level, strong in vitro inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
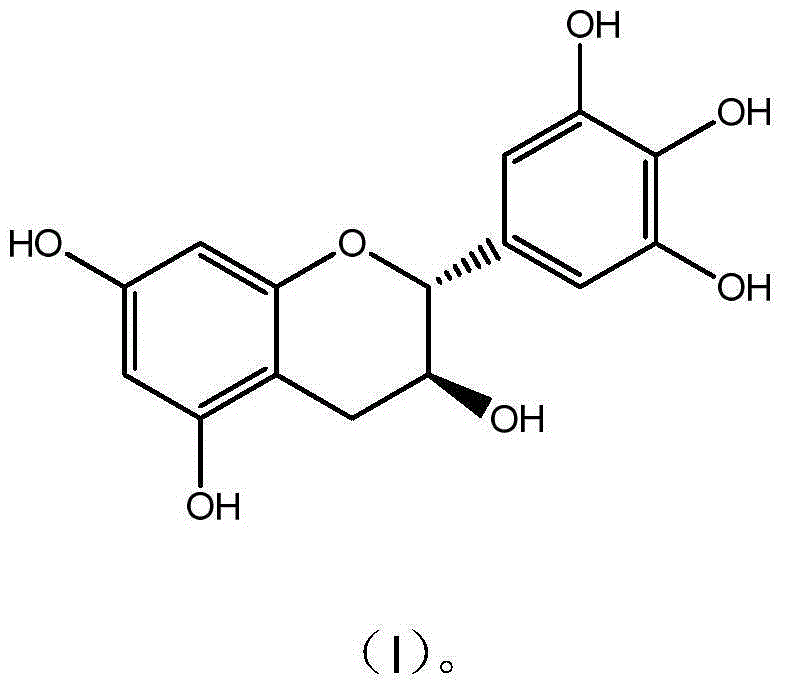
[0025] Preparation and Characterization of Example 1 Gallocatechin GC
[0026] Get 10g epigallocatechin (EGC) and dissolve in 1000mL McIlvaine buffer solution (pH5.0), be placed in autoclave, 120 ℃ of reaction 30 minutes, take a sample after reaction finishes, high performance liquid chromatography analysis result shows that conversion rate 62%; after the reaction solution was concentrated, it was separated and purified by preparative HPLC (C18 chromatographic column 5U, 50*500mm), eluted with a gradient of 10%-95% ethanol, and the GC components with a purity higher than 94% were collected and concentrated Remove part of the ethanol, add 3 times the amount of water and continue to concentrate. When the solution becomes turbid, stop the concentration and place it for overnight crystallization, filter, and freeze-dry the crystals to obtain monomer compound gallocatechin (GC) with a purity of 99.3%. The detection and characterization data are as follows:
[0027] GC C 15 h 14 ...
Embodiment 2
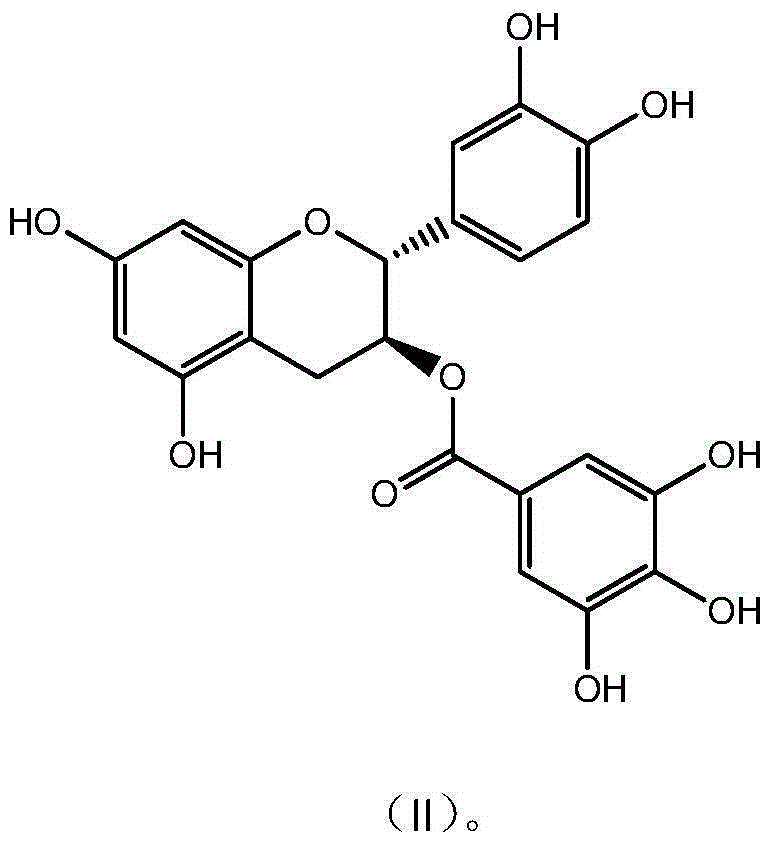
[0030] Example 2 Preparation and Characterization of Catechin Gallate CG
[0031] Get 10g epicatechin gallate (ECG) and dissolve in 1000mL McIlvaine buffer solution (pH5.0), be placed in autoclave, 120 ℃ of reaction 30 minutes, take a sample after reaction finishes, high performance liquid chromatography analysis result shows, The conversion rate was 56%; after the reaction solution was concentrated, it was separated and purified by preparative HPLC (C18 chromatographic column 5U, 50*500mm), eluted with a gradient of 20%-95% ethanol, and the CG component with a purity higher than 94% was collected , Concentrate to remove part of the ethanol, add 3 times the amount of water and continue to concentrate, when the solution becomes turbid, stop concentrating and place it overnight for crystallization, filter, freeze-dry the crystals to obtain the monomeric compound catechin gallate with a purity of 98.9% (CG). The detection and characterization data are as follows:
[0032] CG C ...
Embodiment 3
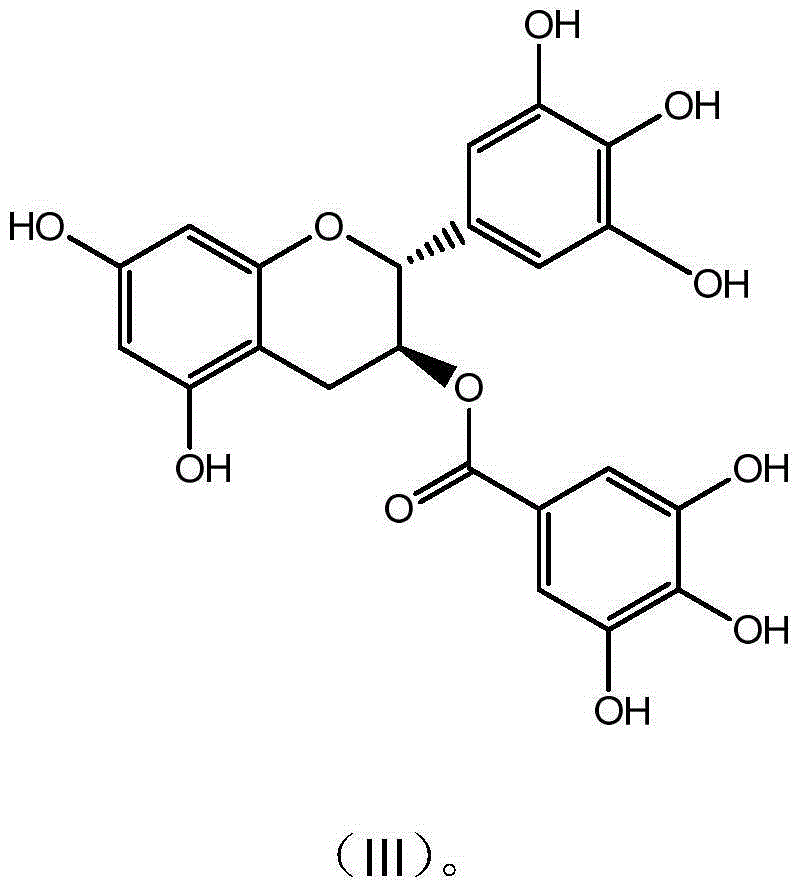
[0035] Example 3 Preparation and Characterization of Gallocatechin Gallate GCG
[0036] Get 10g epigallocatechin gallate (EGCG) and dissolve in 1000mL McIlvaine buffer solution (pH5.0), be placed in autoclave, 120 ℃ of reaction 30 minutes, take a sample after reaction finishes, high-performance liquid chromatography analysis result shows , the conversion rate was 56.5%; after the reaction solution was concentrated, it was separated and purified by preparative HPLC (C18 chromatographic column 5U, 50*500mm), eluted with a gradient of 15%-95% ethanol, and the GCG group with a purity higher than 94% was collected Divide, concentrate to remove part of ethanol, add 3 times the amount of water and continue to concentrate, when the solution becomes turbid, stop the concentration and place it overnight for crystallization, filter, freeze-dry the crystals to obtain the monomer compound gallocatechin gallate with a purity of 99.1% acid ester (GCG). The detection and characterization dat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com