Guaialactone compound as well as preparation method and application thereof
A technology of ester compound and guaiac, which is applied in the field of guaiac lactone compound and its preparation, and achieves the effect of novel structure and strong inhibitory effect in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Instruments and materials:
[0029] AV-400 MHz superconducting nuclear magnetic resonance instrument (Bruker, Germany); SolariX 7.0T high-resolution Fourier transform mass spectrometer (Bruker, Germany); Nicolet iS50R Fourier transform infrared spectrometer (ThermoScientific, USA); 2535 preparative high-efficiency Liquid chromatograph (Waters, USA); HPLC column XBridgeTM Prep C18 column (250 mm×19 mm, 5 μm) (Waters, USA); 1260 analytical high performance liquid chromatography (Agilent, USA); Analytical HPLC column XBridgeTM Prep C18 column (250 mm × 4.6 mm, 5 μm) (Waters, USA); Milli-Q Integral 5 pure water instrument (Merck Millipore, Germany); R-210 rotary evaporator ( Swiss Büchi Company); miVac Quattro Vacuum Centrifugal Concentrator (GeneVac, UK); Deuterium Reagent: CDCl3 (CIL, USA); Chromatographically Pure Methanol (Thermo Fisher Scientific, USA); Chromatographically Pure Tetrahydrofuran (Shanghai Xingke High Purity Solvent Co., Ltd. company); Sephadex LH-20 dex...
Embodiment 2
[0041] Structural identification of compound 1 and compound 2:
[0042] Compound 1: White needle-like crystal, easily soluble in chloroform. IR (KBr, cm -1 ) vmax: 2922, 1764, 1748, 1693, 1616, 1228, 1153, 1031, 959; HR-ESI-MS shows that the molecular formula is C 17 h 20 o 5 (calcd.forC 17 h 20 NaO 5 [M+Na] + 327.12029, found327.12017), with an unsaturation of 8. 13CNMR and DEPT spectra show that the compound structure contains 17 carbon signals, including 3 CH 3 , 2 CH 2 , 7 CHs and 5 Cs (see Table 1). The γ-lactone (1764cm -1 ), α, β-unsaturated carbonyl (1693cm -1 ) and double bond (1616cm -1 ) characteristic signal. 1 The two doublet signals shown in the HNMR spectrum [δ H 6.35 (1H, d, J=3.4Hz)] and [δ H 5.84 (1H, d, J=3.4Hz)] is the characteristic absorption peak of methylene hydrogen outside the ring of α-methylene-γ-sesquiterpene lactone. 1HNMR spectrum also shows a doublet methyl hydrogen [δ H 0.95 (3H, d, J=7.6Hz)], a vinyl methyl hydrogen [δ H 2.3...
Embodiment 3
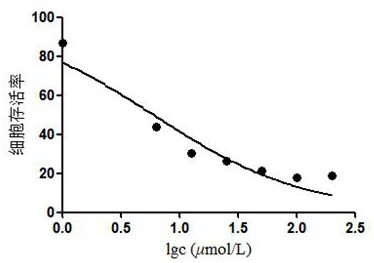
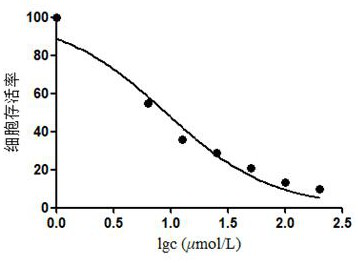
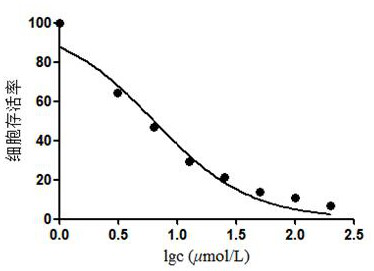
[0049] Biological activity test of compound 1 and compound 2:
[0050] MTT method was used to test the effect of compounds 1 and 2 on the proliferation of human liver cancer cell line HepG2, and cisplatin was used as a positive control.
[0051] The specific test method is: in a 10cm culture dish, culture the cells to the logarithmic growth phase, digest with 1mL trypsin and centrifuge to collect the cells, count the cells on a cell counting plate and dilute the cells to 5×10 4 cells / mL, inoculated into 96-well plate, 100 μL per well, cultured overnight at 37°C in a 5% CO2 incubator, and then added to each well the sample to be tested diluted with the medium to 2 times the final concentration, so that the final concentrations of the drugs were respectively 6.25, 12.5, 25, 50 and 100 μmol L -1 , set 4 duplicate wells for each concentration, and after continuing to culture for 24 hours, add 20 μL of MTT solution (0.5% MTT) to each well, and continue to cultivate for 4 hours. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com