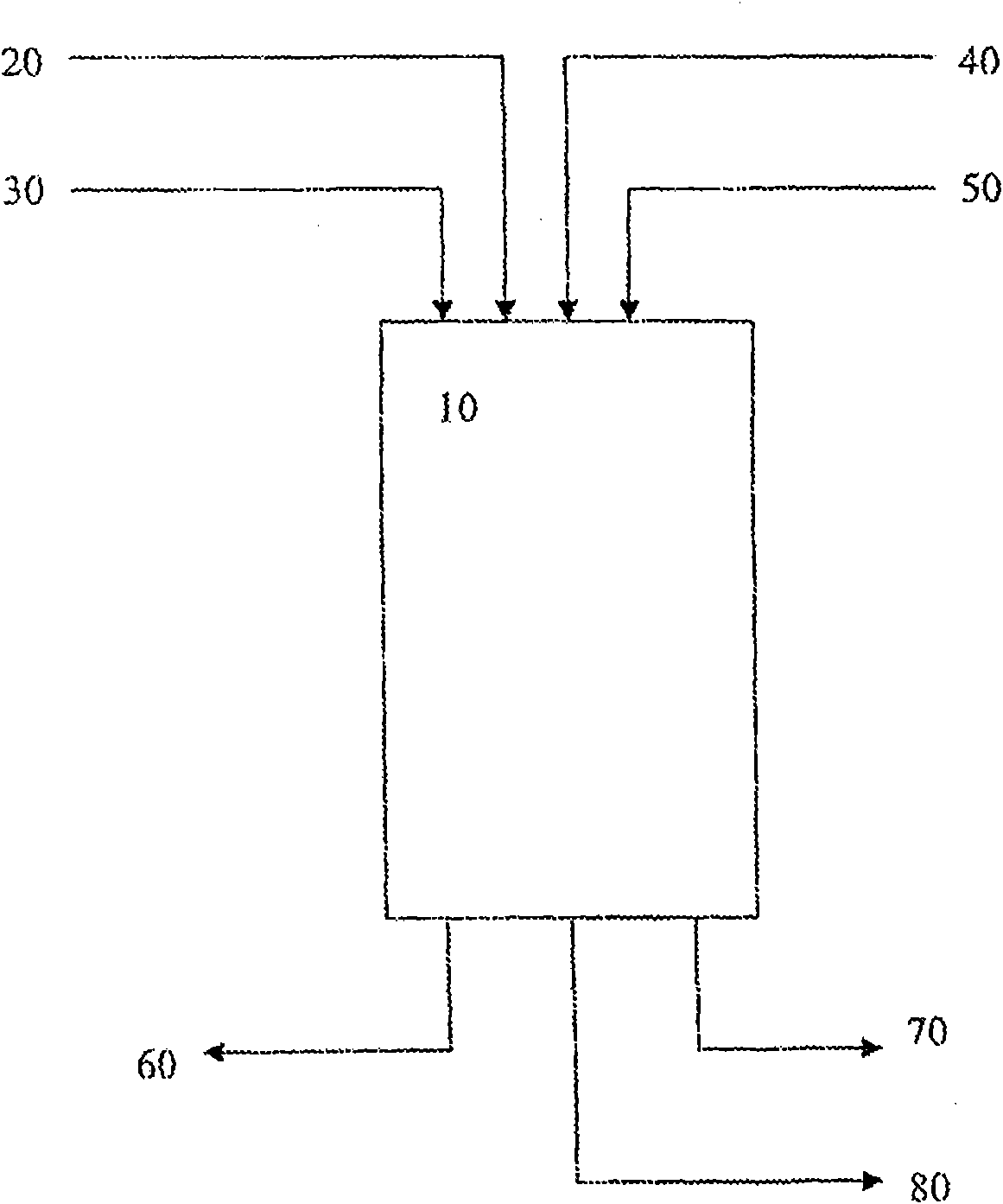
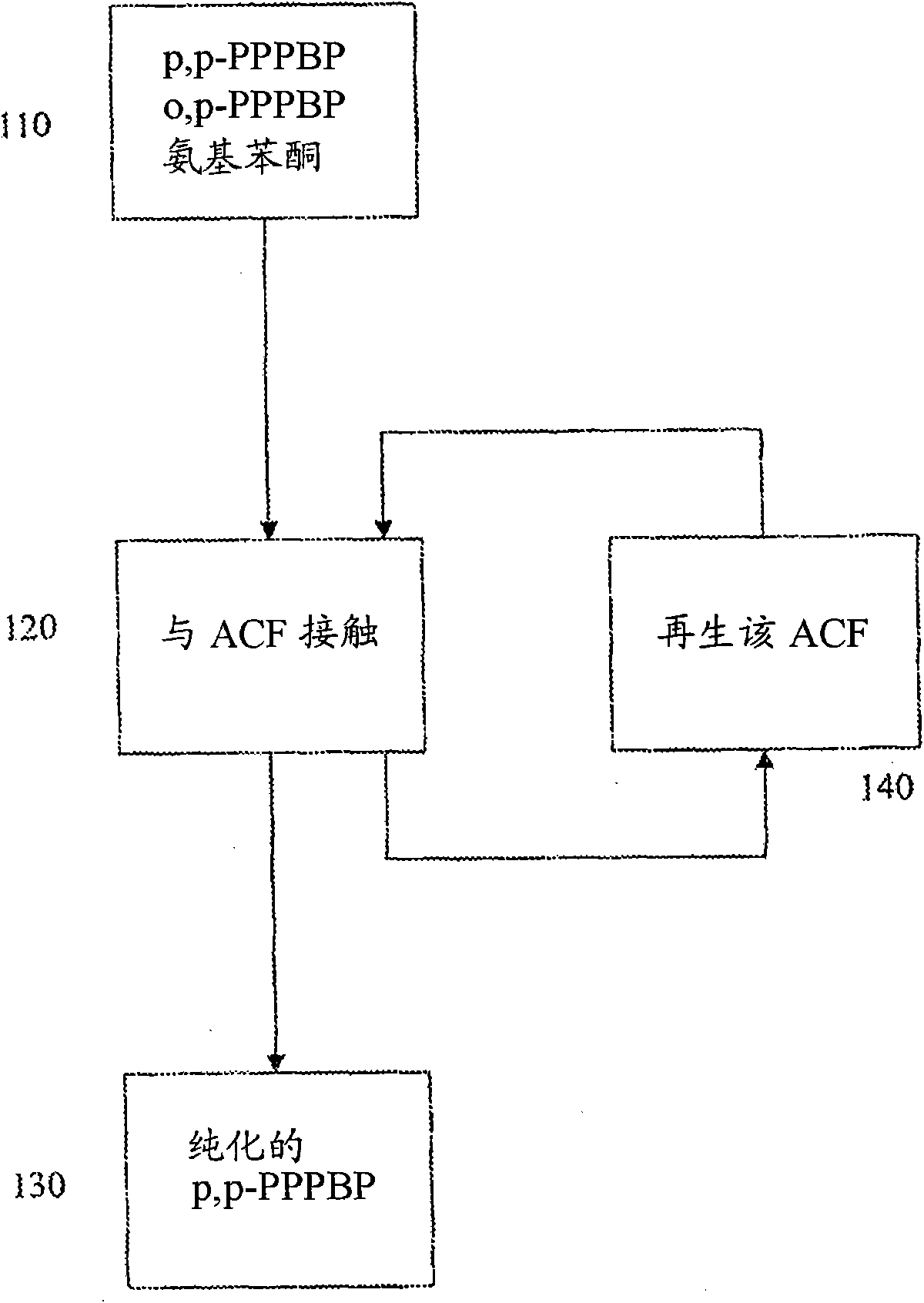
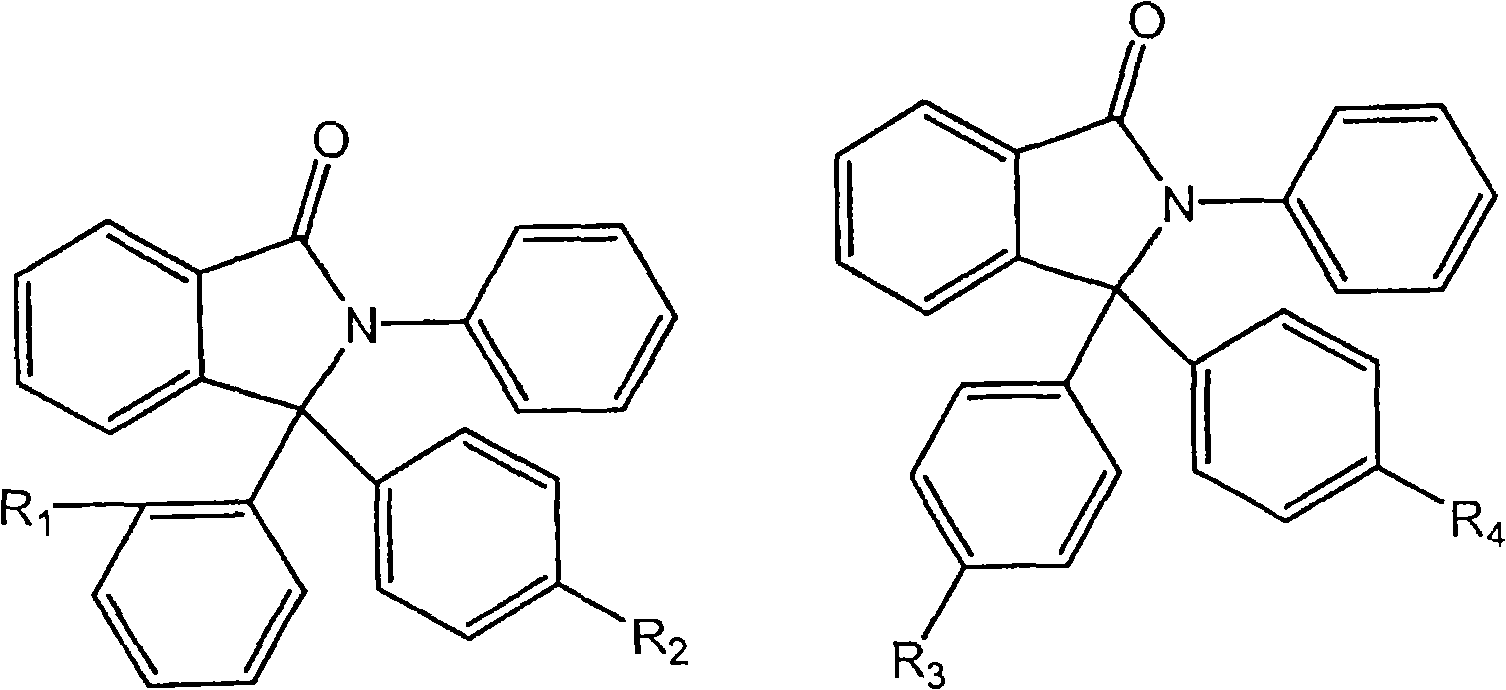
Process for purifying 2-phenyl-3, 3 -bis(4-hydroxyphenyl) phthalimidine (PPPBP)
A technology of pyrrolidone and phenyl, which is applied in the field of purifying 2-phenyl-3, and can solve the problems of product loss and high cost of PAC
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Activated carbon fibers obtained from two different vendors, Anshan and Sutong, were pretreated as described above. Prepare 4 flasks; each flask contains approximately 2.0 grams of ACF. Two flasks contained ACF from Anshan and two flasks contained ACF from Sutong.
[0059] 200.0 mL of the pre-purification solution was placed in each flask. The flask was then shaken for 2 hours. Two flasks (each from a different vendor) were shaken at 20°C and the other two at 70°C. The solution was then analyzed by HPLC to measure the concentration change of aminophenone. Aminophenone had an initial peak area of 175,537. The results are shown in Table 1.
[0060] Table 1.
[0061]
sample
seller
temperature
The purified peak
area
Removal ratio (%)
1
Anshan
20℃
170,989
2.59
2
Anshan
70℃
169,914
3.20
3
Sutong
20℃
147,772
...
Embodiment 2
[0068] Activated carbon fibers from Sutong were pretreated as described above. Prepare three flasks; each flask contains 2.0 grams of ACF. 200.0 mL of the pre-purification solution was placed in each flask. The flasks were then shaken for 1, 2, and 3 hours, respectively. Each solution was then analyzed by HPLC to measure the change in concentration of aminophenone. The initial peak area for aminophenone was 32,770. The results are shown in Table 3.
[0069] table 3.
[0070] sample
[0071] Because ACF has a high adsorption rate, additional contact does not increase the removal ratio. Under the static adsorption regime shown here, a two hour exposure time was sufficient to remove most of the aminophenone impurity.
Embodiment 3
[0073] Activated carbon fibers from Sutong were pretreated as described above. Prepare 1 flask; it contains 2.0 grams of ACF. 200.0 mL of the pre-purification solution was placed in the flask, and the flask was shaken at 70°C for 2 hours. The solution was then analyzed by HPLC to measure the concentration changes of p,p-PPPBP, aminophenone and phenolphthalein (PP). The results are shown in Table 4.
[0074] Table 4.
[0075] component
[0076] This data indicates that ACF has excellent selective adsorption of aminobenzophenone compared to p,p-PPPBP and PP. ACF also removes p,p-PPPBP in a smaller proportion than PAC (7-8%), so that less of the desired product is lost during purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com