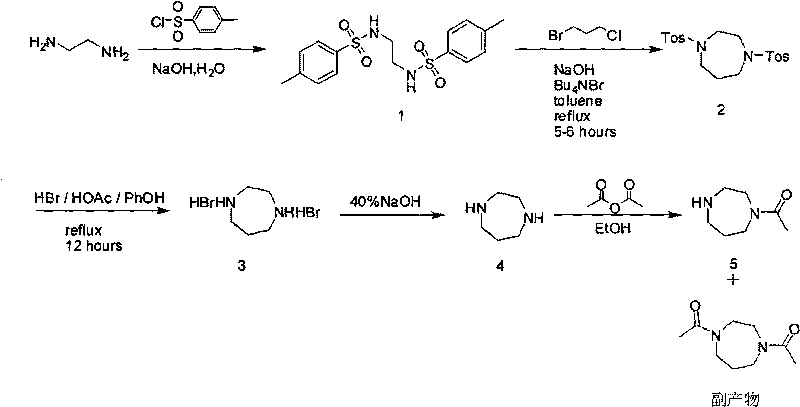
Method for producing monoacetylated homopiperazine
A technology for monoacetyl homopiperazine and homopiperazine, which is applied in the field of producing monoacetyl homopiperazine, can solve problems such as being difficult to realize industrialization, and achieve the effects of easy operation, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0031] Example 1
[0032] Intermediate product of the first step 1: At 10-20℃, add ethylenediamine (70g, 1.17mol) in water (163ml) to a 2L four-neck flask, stir, and add sodium hydroxide to the reaction system Aqueous solution (114g, 2.85mol, 746ml of water), when the temperature is lowered by 10-20°C, add p-toluenesulfonyl chloride (496.6g, 2.6mol) in batches, after the addition, stir for 5-6 hours. Filter for 1-2 days (although the reaction solution is viscous and difficult to filter, but try to filter because there are inorganic salts in the solution), the filter cake is slurried with ethanol at 60℃, stirred for 30 minutes, and when the temperature is cooled to room temperature, filter again. The cake was washed with ethanol and dried to constant weight by infrared to obtain a white solid intermediate product (318 g) with a yield of 72.6%.
[0033] Intermediate product 2: Intermediate product 1 (36.8g, 0.1mol), tetrabutylammonium bromide (8g, 0.025mol), toluene (400ml), 5% sodi...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap