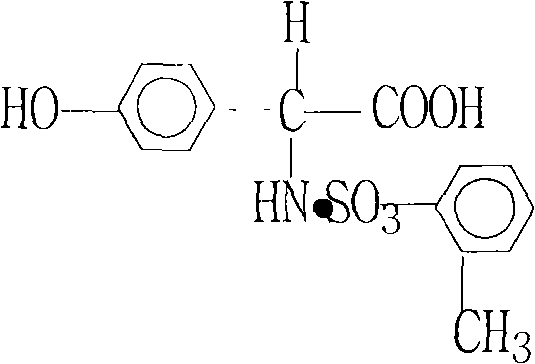
Technology for splitting DL-p-hydroxyphenylglycine-o-toluenesulfonate
A technology of hydroxyphenylglycine o-toluenesulfonate, which is applied in the field of DL-p-hydroxyphenylglycine o-toluenesulfonate separation technology, can solve the problem of no market competitiveness, low yield, and increased labor costs. Labor intensity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] 1. First add 3000 kg of pure water into the 5000L reactor, start stirring, then put 1363 kg of DL-p-hydroxyphenylglycine o-toluenesulfonic acid hydrochloride, and open the steam valve to raise the temperature.
[0015] 2. Raise the temperature to 85°C and keep it at 85-90°C for 1 hour.
[0016] 3. Add 30 kg of activated carbon and decolorize, 120 kg of D-(-)-p-hydroxyphenylglycine o-toluenesulfonate, keep warm at 90-95°C for 30 minutes and filter while hot.
[0017] 4. Cool down the temperature of the filtered mother liquor to 28°C and start to measure the optical rotation. When the optical rotation is ≥+2°, discharge and centrifuge.
[0018] 5. D-(-)-p-hydroxyphenylglycine o-toluenesulfonate obtained after centrifugation: 230-250 kg, optical rotation ≥ -55°.
[0019] 6. After centrifugation, put the mother liquor into the output D-(-)-p-hydroxyphenylglycine o-toluenesulfonate equal weight of DL-p-hydroxyphenylglycine o-toluenesulfonate, after heating up and completely...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com