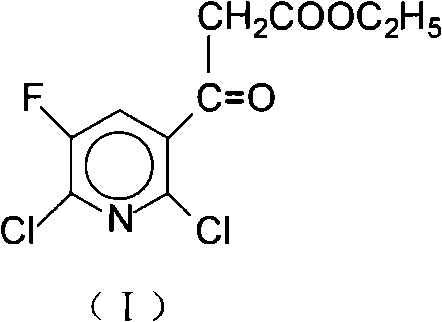
Method for preparing 2, 6-dichloro-5-fluorine nicotinoyl ethyl acetate
A technology of ethyl flunicotinyl acetate and acid hydrolysis, applied in the direction of organic chemistry, can solve the problems of low total yield, few preparation steps, large melting range, etc., achieve good process reproducibility, and avoid hydrolysis side reactions , the effect of improving the total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of 2,6-dichloro-5-fluoronicotinoyl ethyl acetate (I)A
[0032] The specific operation steps are as follows:
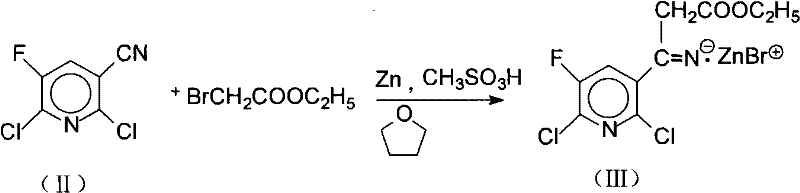
[0033] ① Generate zinc salt intermediate 3-(2,6-dichloro-5-fluoro-3-pyridyl)-3-iminopropionic acid ethyl ester zinc bromide (III)
[0034] 4.12g (63.2mmol) of zinc powder was added to 60.0mL of tetrahydrofuran and 24.0mg of methanesulfonic acid solution, and the mixture was refluxed and stirred for 1 hour to activate the zinc powder, and 8.0g (41.9mmol) of 2,6 - Dichloro-5-fluoro-3-pyridinecarbonitrile (II), followed by the dropwise addition of 8.8 g (52.7 mmol) ethyl bromoacetate within 1 hour. After the addition, the reaction mixture continued to reflux and stir for 0.5 to 2 hours to generate the reaction solution containing the zinc salt intermediate (III), for subsequent use. The main chemical reactions involved are shown in the following formula:
[0035]
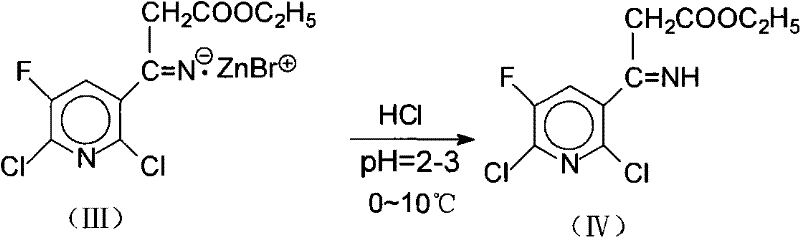
[0036] ②Acidification of zinc salt intermediate (III) to generate imidoest...
Embodiment 2
[0042] Example 2 Preparation of 2,6-dichloro-5-fluoronicotinoyl ethyl acetate (I) B
[0043] The specific operation steps are as follows:
[0044] Step 1. and step 2. are identical with embodiment 1;
[0045] Step ③ When preparing 2,6-dichloro-5-fluoronicotinoyl ethyl acetate (I), add 3-4 mL of concentrated sulfuric acid dropwise to carry out acid-catalyzed alcoholysis, instead of adding hydrogen chloride dropwise in step ③ in Example 1 6~7mL of dehydrated ethanol solution carried out acid-catalyzed alcoholysis, and the rest were all the same as step 3 in Example 1 to obtain 2,6-dichloro-5-fluoronicotinoyl acetate (I)B fine work, and its yield It is 95%, the purity is 99%, and the melting point mp is 68°C to 70°C (decomposition).
Embodiment 3
[0046] Example 3 Preparation of 2,6-dichloro-5-fluoronicotinoyl ethyl acetate (I)C
[0047] Step 1. When the zinc salt intermediate (III) is generated, activate the zinc powder by stirring the mixture for 0.5 hours under reflux, instead of 1. the mixture in step 1 of Example 2 activates the zinc powder by stirring the mixture under reflux for 1 hour, and the rest are all the same as in step 1 of Example 2. same;
[0048] Step ② and step ③ are all the same as in Example 2, and 2,6-dichloro-5-fluoronicotinoyl acetate (I)C fine product is obtained, the yield is 90%, the purity is 99%, and the melting point mp is 68℃~70℃ (decomposition).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com