Formulations for treatment of ocular diseases or conditions
A technology of ocular diseases, therapeutic agents, applied in the field of preparations for the treatment of ocular diseases or conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-45
[0554] Example 1-45 - Preparation and Administration of Compositions Containing Rapamycin
[0555] The preparation and characterization of exemplary rapamycin-containing compositions, and their administration to New Zealand white rabbits are described in Mudumba et al., US 2006 / 0258698 A1 and WO 2006 / 102378 A2 (Examples 1-45 and its associated drawings are incorporated herein by reference).
Embodiment 46
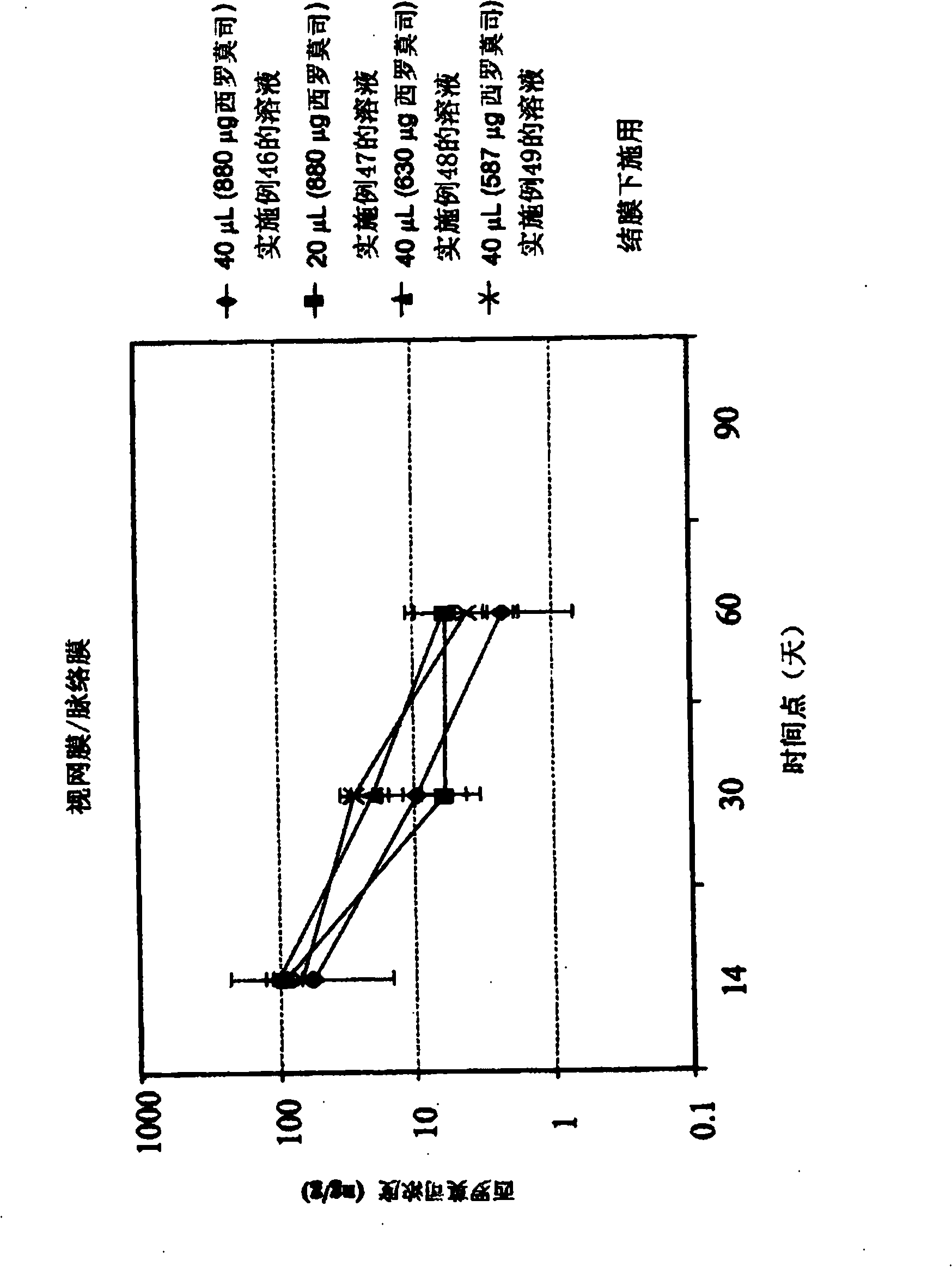
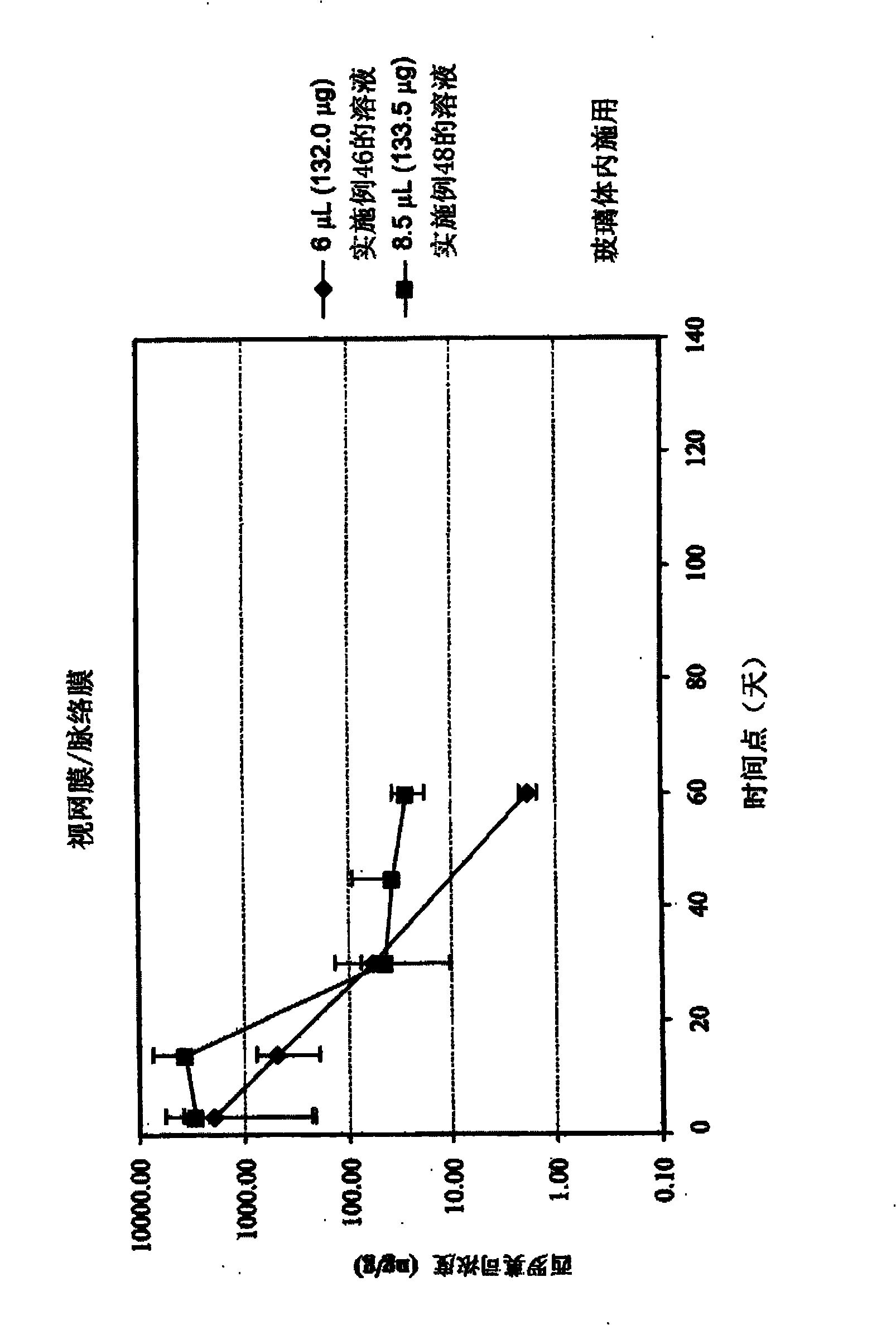
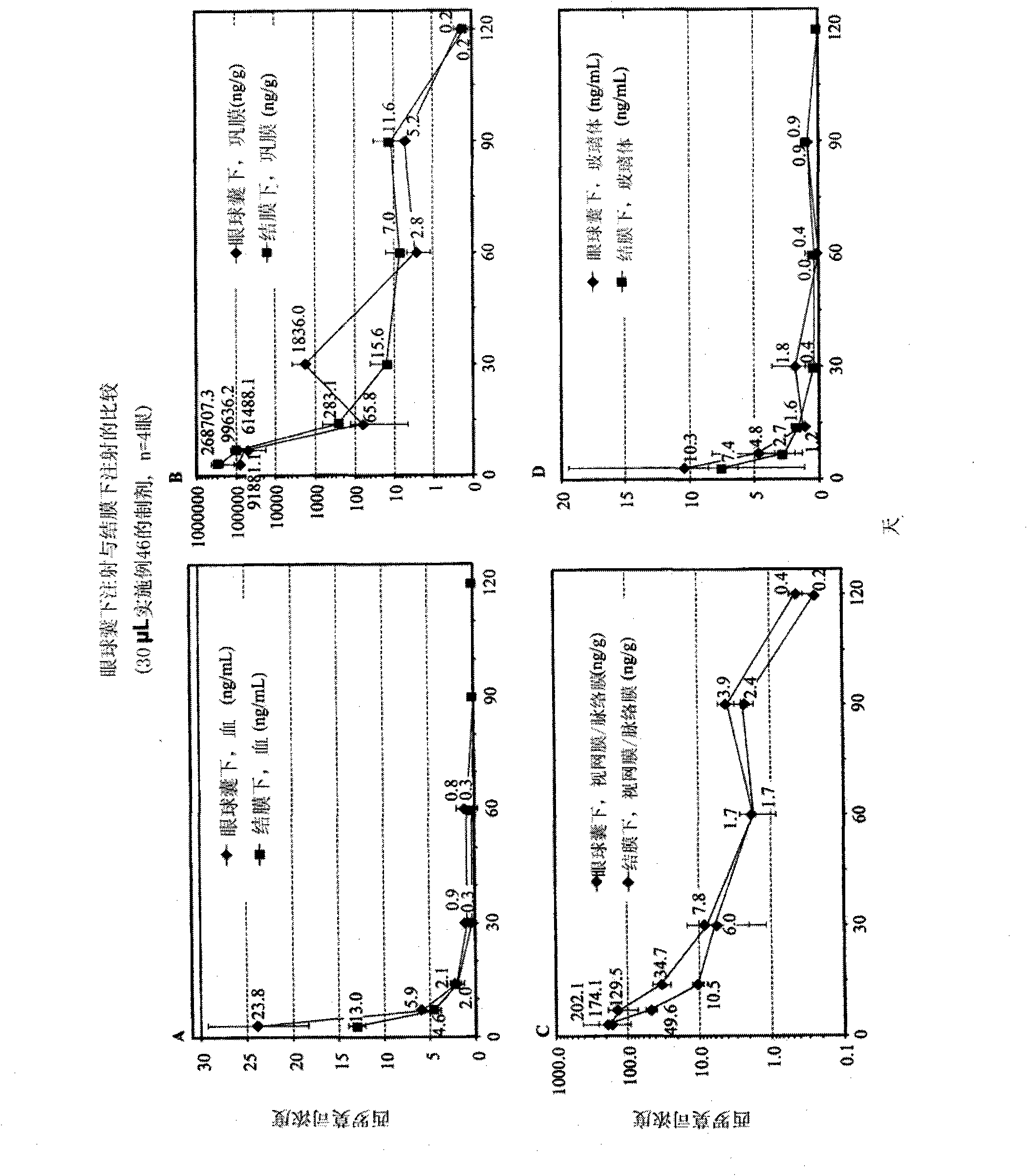
[0556] Example 46 - Preparation and Characterization of Solutions Containing Rapamycin
[0557] About 320g ethanol with N 2 Rinse for about 10 minutes, then add about 40 g of sirolimus to the ethanol. The mixture was sonicated for about 20 minutes, at the end of which all the sirolimus had gone into solution, forming a stock solution of sirolimus. The dilution solvent was prepared by sonicating about 1880 g of PEG 400 for about 60 minutes, then flushing the solvent with nitrogen for about 10 minutes.
[0558] The sirolimus stock solution and PEG 400 were then rotated at about room temperature for about 10 minutes in a rotary evaporator to mix the stock solution and dilution solvent. After mixing the solution was flushed with nitrogen for about 10 minutes and blanketed with nitrogen for about 5 minutes. After the solution was flushed and flooded with nitrogen, about 240 g of excess ethanol was evaporated from the solution by increasing the temperature of the solution, mainta...
Embodiment 47
[0560] Example 47 - Preparation and Characterization of Solutions Containing Rapamycin
[0561] About 32g ethanol with N 2 Rinse for about 10 minutes, then add about 4 g of sirolimus to the ethanol. The mixture was sonicated for approximately 20 minutes, at the end of which all the sirolimus had gone into solution, thereby forming a stock solution of sirolimus. The dilution solvent was prepared by sonicating about 92 g of PEG 400 for about 60 minutes, then flushing the solvent with nitrogen for about 10 minutes.
[0562] The sirolimus stock solution and PEG 400 were then rotated at about room temperature for about 10 minutes in a rotary evaporator to mix the stock solution and dilution solvent. After mixing the solution was flushed with nitrogen for about 10 minutes and blanketed with nitrogen for about 5 minutes. After the solution was flushed and flooded with nitrogen, about 28 g of excess ethanol was evaporated from the solution by increasing the temperature of the solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com