Application of
The technology of ester compound and toad is applied in the application field of bufa lactone compound in the research and development of new anti-tumor drugs, and can solve the problems of complex composition, allergic reaction, no Hellebogen and its derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of Bufadienolactone Extract
[0021] Get 10Kg of toad skin and extract 3 times with 95% (v / v) ethanol, the amount of alcohol used for each extraction is 10 times the weight of the sample toad skin, and the extraction time is 120 minutes each time, the filtrate is combined, and the filtrate is concentrated under reduced pressure to 10L , on XAD-4 macroporous resin (Rohm and Haas), first eluted with 3 times column volume of water, then eluted with 3 times column volume of 30% ethanol (v / v), and then eluted with 3 times column volume 95% ethanol (v / v) was eluted, the latter was collected, the solvent was recovered under reduced pressure, and dried to obtain 125 g of bufadienolactone extract.
Embodiment 2
[0022] Example 2: Preparation of helleboregenin
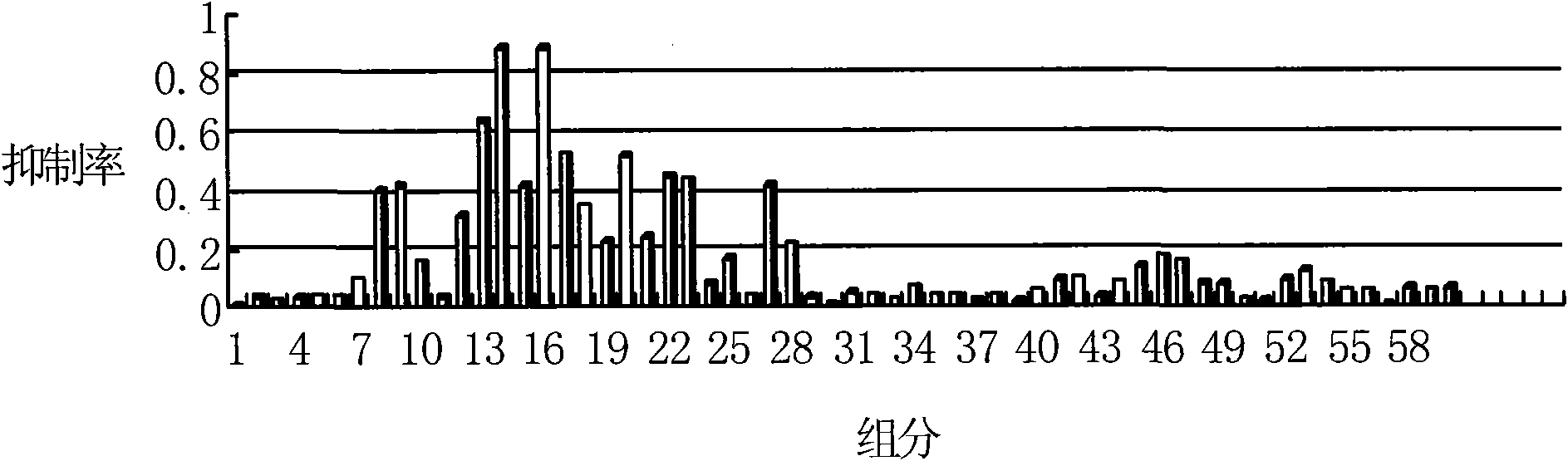
[0023] 75 g of the bufadienolactone extract obtained in Example 1 was separated by preparative HPLC (Waters Xterra C18, A: 0.1% (v / v) formic acid water; B: 0.1% (v / v) formic acid acetonitrile, Gradient conditions: 5% B ~ 25% B, 30min; 25% B ~ 50% B, 22min; 50% B ~ 95% B, 18min; 95% B, 5min, 300nm), from the fifth minute to the 65th minute , the effluent per minute is taken as a component, and a total of 60 components (Fr.1~Fr.60) are collected.
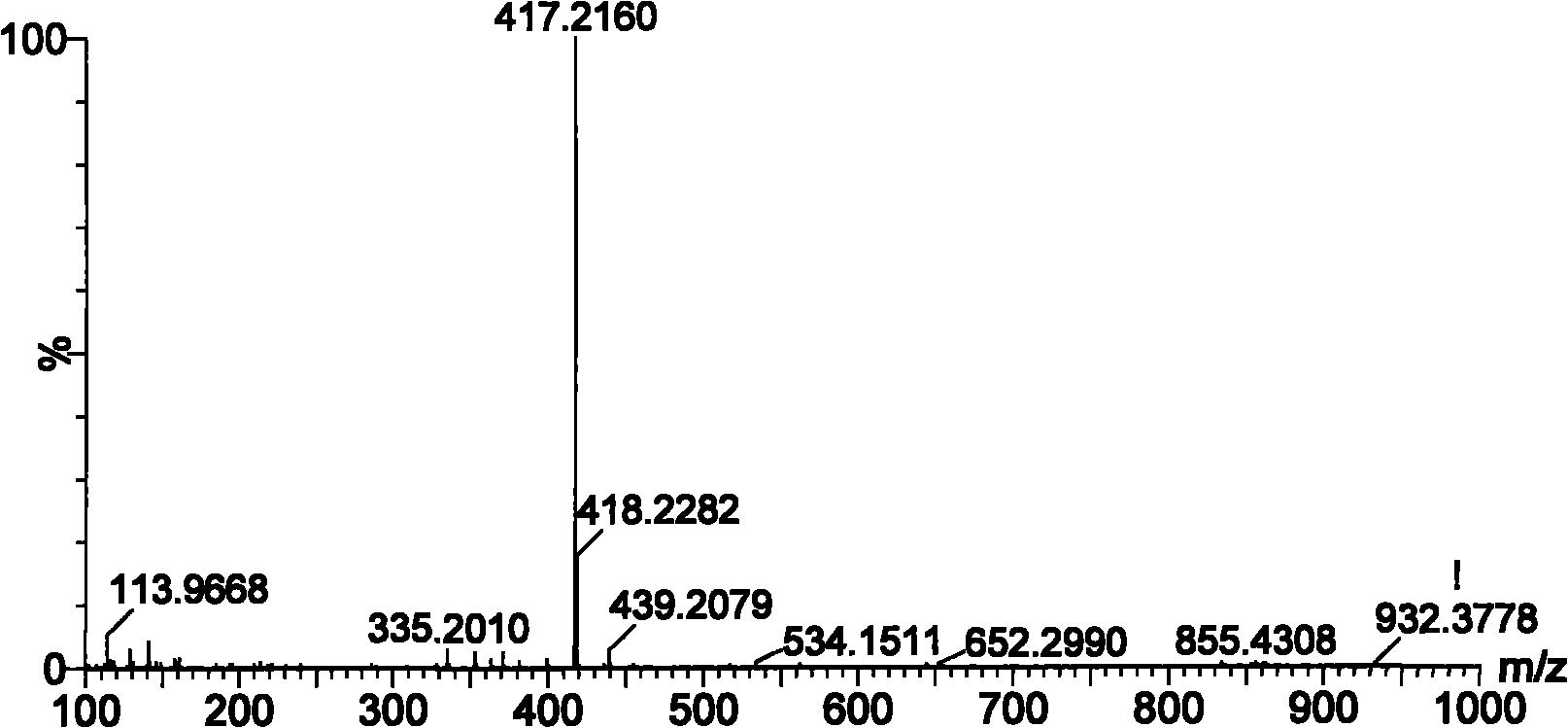
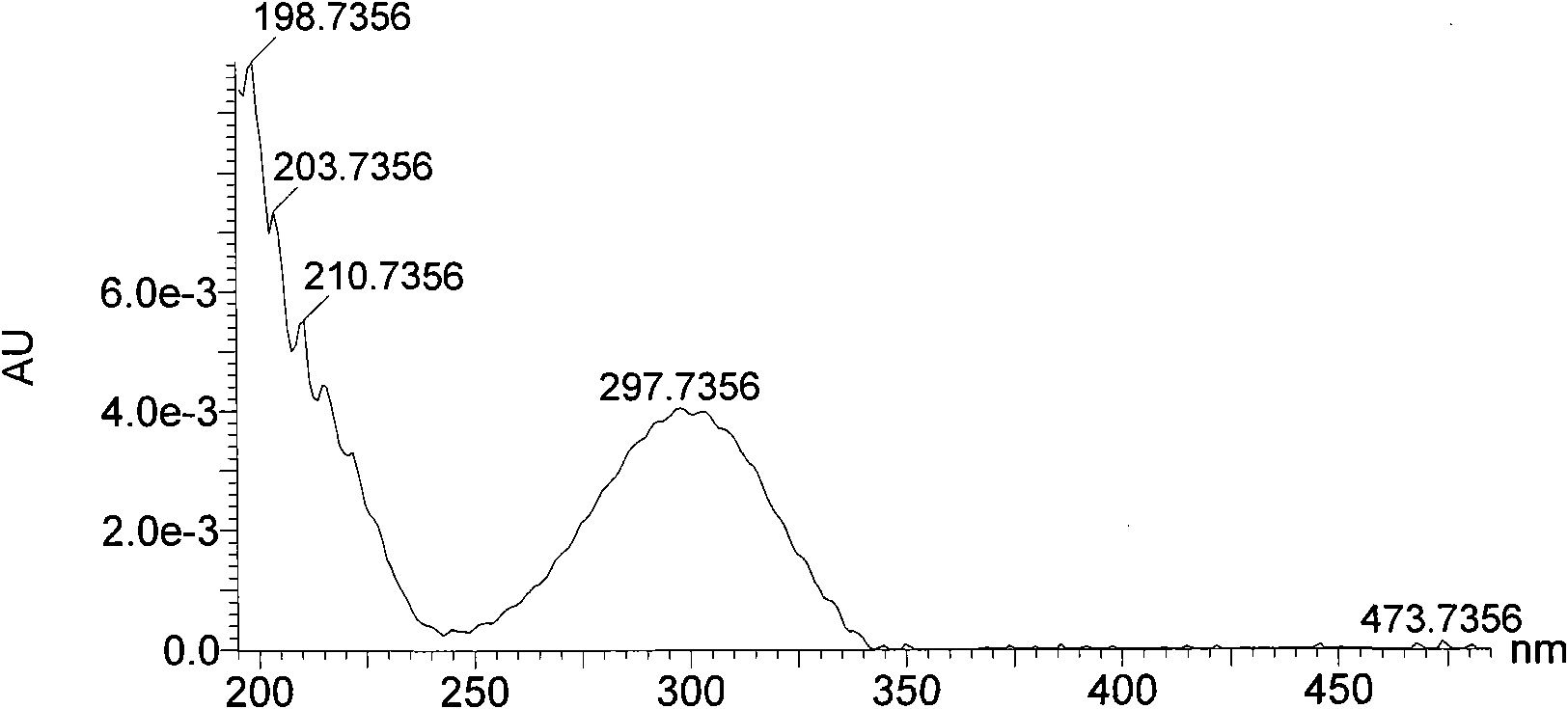
[0024] Fr.13 recovered solvent, separated by HPLC (the chromatographic column is based on silica gel, and the functional ligand is β-cyclodextrin), A: 0.1% formic acid water; B: 0.1% formic acid acetonitrile, gradient conditions: 95% B to 60% B, 40min, 300nm), monitor with ultraviolet, collect chromatographic peak, reclaim solvent, obtain compound I 11mg, HPLC detection content is 99%, its NMR, mass spectrometry, ultraviolet data see Table 1, Table 2, figure 1 , figure 2 , accordin...
Embodiment 3
[0029] The cytotoxicity of embodiment three helleboregenins to SMMC-7721 and A549
[0030] Materials SMMC-7721 and A549 cell lines were purchased from the Second Military Medical University in China
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com