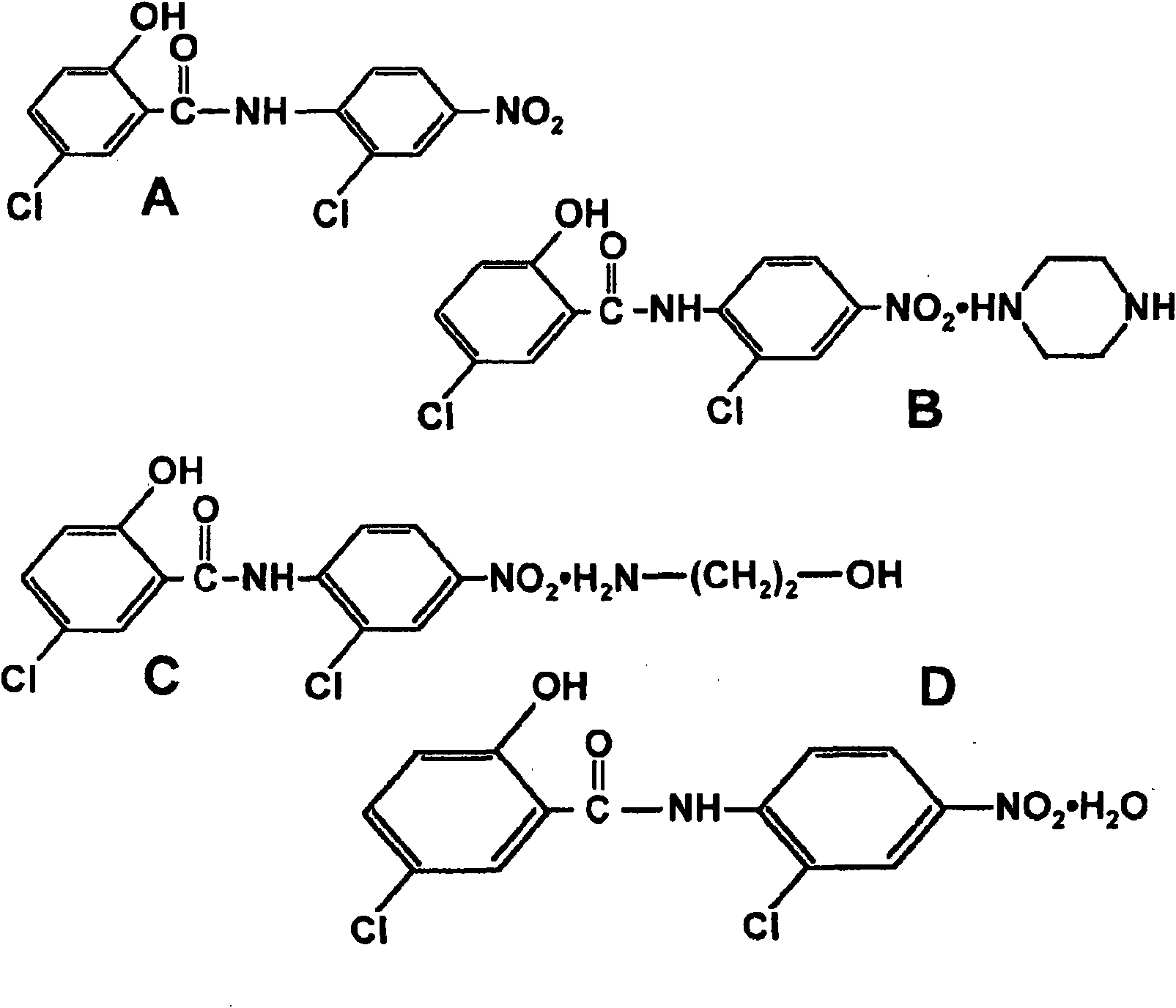
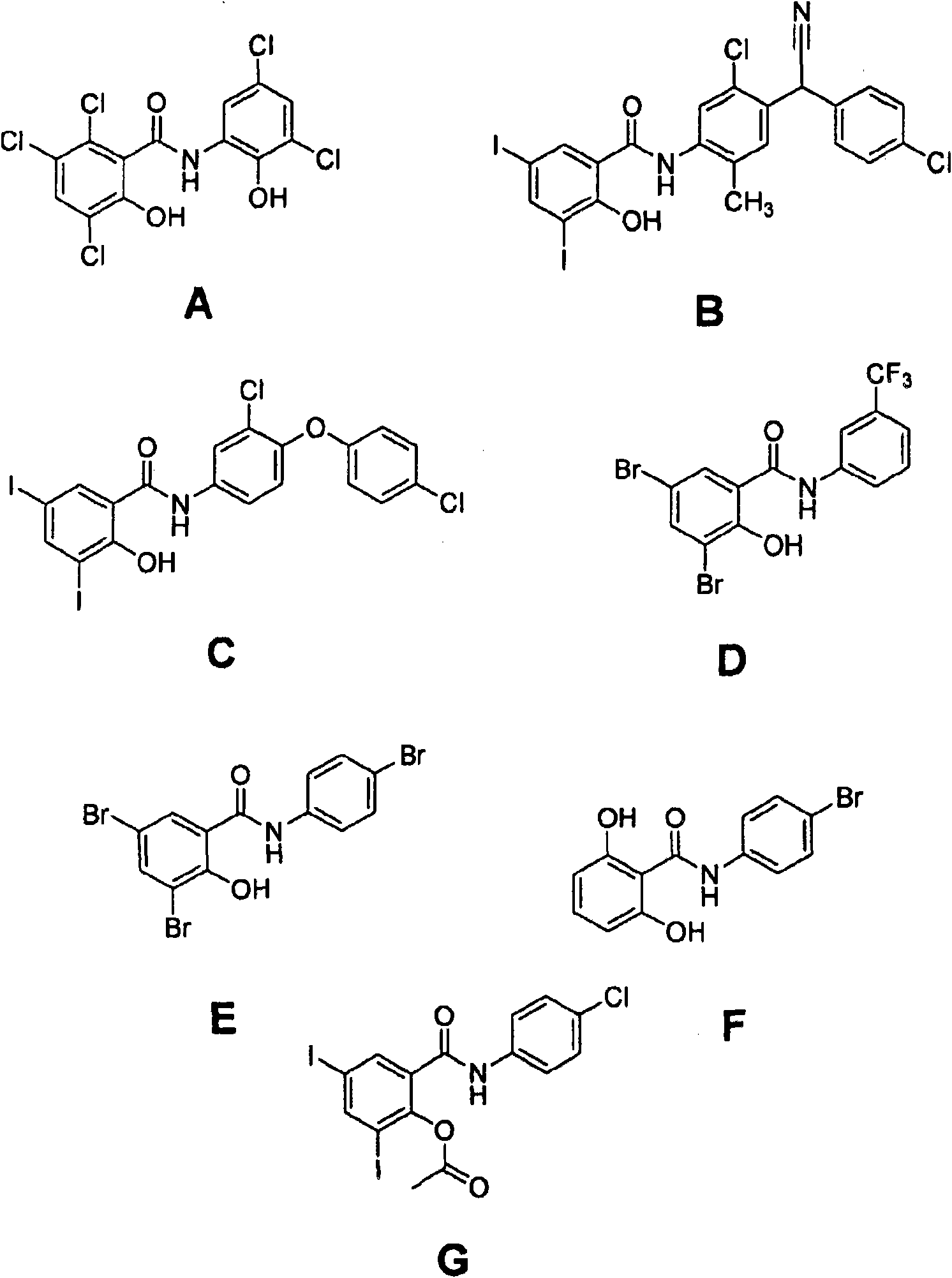
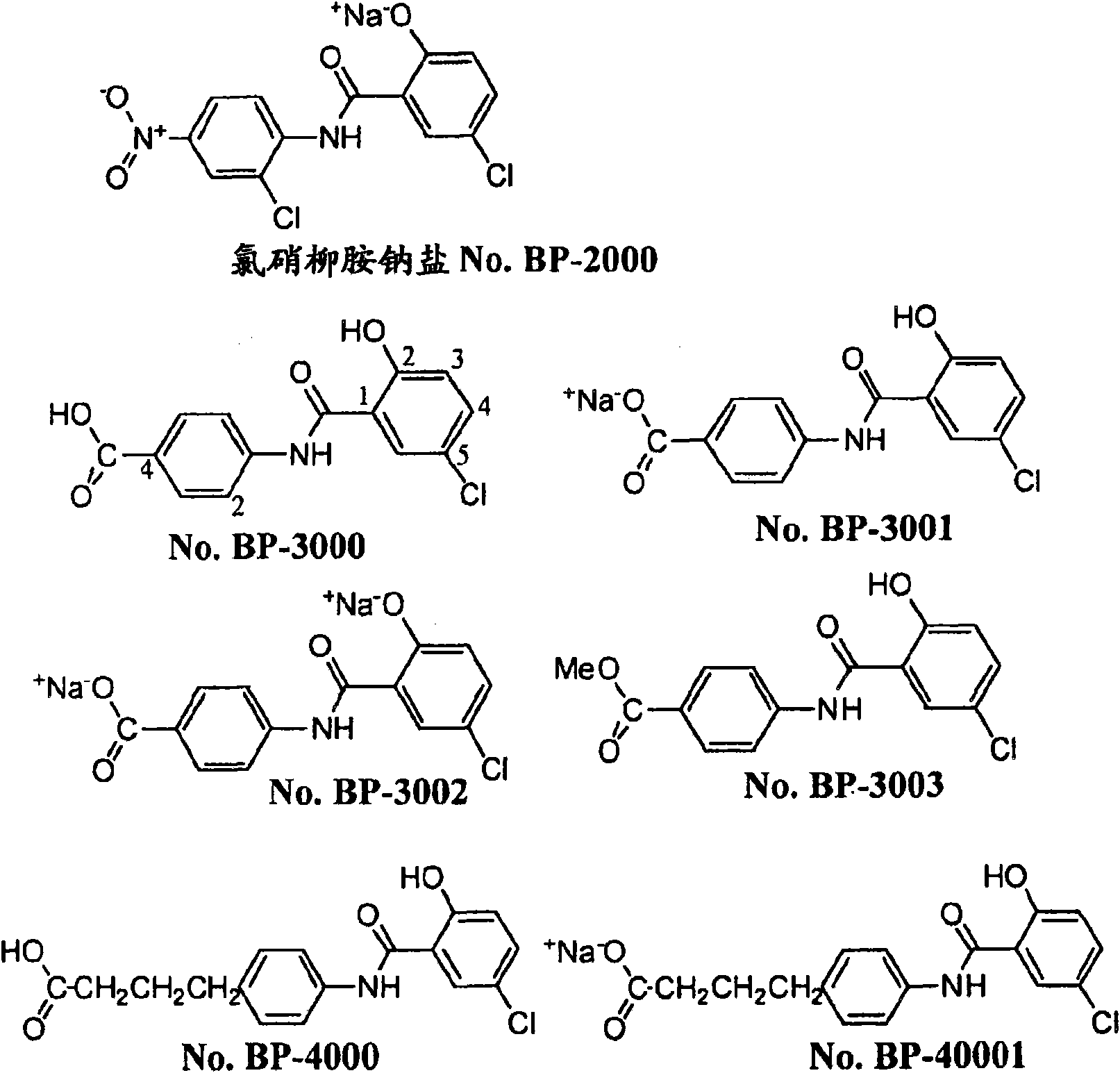
Salicylanilide modified peptides for use as oral therapeutics
A technology of salicylanilide, a therapeutic peptide, applied in the field of enhancing the bioavailability of this peptide compound, which can solve problems such as discomfort, inconvenience to patients, and non-compliance of patient treatment plans
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0455] Niclosamide increases uptake / bioavailability of orally administered peptides
[0456] We previously reported that the amino acid sequence D-W-F-K-A-F-Y-D-K-V-A-E-KF-K-E-A-F (SEQ ID NO: 5) (see, e.g., U.S. Patent 6,933,279) with at least one protecting group when synthesized from all L-amino acids (L-4F) and to small Rapidly degraded and did not significantly alter the protective ability of HDL to inhibit LDL-induced monocyte chemotactic activity in cultures of human arterial wall cells when administered orally to mice (Navab et al. (2002) Circulation 105:290-292).
[0457]The surprising finding of the present invention is that oral administration of L-4F to mice together with niclosamide resulted in a significant improvement in the ability of HDL to inhibit LDL-induced chemotactic activity of monocytes in these mice. In contrast, oral administration of either agent alone was ineffective or significantly less effective.
[0458] Such as Figure 8 As shown, the combinat...
Embodiment 2
[0473] Salicylanilide in combination with L-4F enhances the formation of pro-βHDL
[0474] Niclosamide plus L-4F results in the formation of pre-βHDL in apoE-null mice after oral administration (see, e.g. Figure 18 ). D-4F (free base) was dissolved in ammonium bicarbonate buffer pH 7.0 containing 0.1% Tween 20 (ABCT). L-4F (free base) and niclosamide were dissolved in ABCT at a ratio of 1:10 (L-4F:niclosamide; weight:weight). Combine ABCT alone or with Figure 18ABCT of micrograms of L-4F or D-4F indicated in the middle x-axis with or without niclosamide indicated in 100 μL by gastric tube to overnight fasted 8-month-old female apoE-deficient mice (n=8 per group) administration. Mice were bled 30 to 40 minutes later and the percentage of apolipoprotein A-I contained in pre-β-1 HDL was determined by scanning in triplicate 2-dimensional gels. Data shown are mean ± standard deviation.
[0475] It was also surprising to find that oral co-administration of niclosamide and L-...
Embodiment 3
[0484] Niclosamide enhances L-4F uptake in ApoE-deficient mice
[0485] use 14 C-L-4F Determines L-4F uptake with and without niclosamide (BP-124). Fasted 6-month-old female apoE-null mice (n=4 per group) were administered 100 μg chloride in 200 μL pH 7.0 ammonium bicarbonate, 0.1% Tween 20 with or without gastric tube Nisamide L-4F (contains 10 micrograms of L-4F per mouse at 21,000 dpm). continue fasting and Figure 27 Mice were bled at the time points indicated on the middle x-axis and the dpm per mL of plasma was determined. Figure 27 The area under the curve (AUC) for mice receiving L-4F+niclosamide was 4.4 times higher than for mice receiving L-4F without niclosamide.
[0486] The data suggest that one of the mechanisms by which niclosamide enhances the bioactivity of L-4F in vivo is by enhancing the uptake of L-4F.
[0487] The above data (Examples 1, 2 and 3) show that the combination of niclosamide or other salicylanilides with L-4F and presumably other therapeu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com