Substituted piperazine N-ethyl sulfonamide derivative and preparation and application thereof
A technology of sulfonamide and derivatives, applied in the fields of medicinal chemistry and pharmacology, can solve the problem of low selectivity of PKB
Inactive Publication Date: 2010-10-06
ZHEJIANG UNIV
View PDF2 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, since these compounds have effects on a variety of targets, the selectivity for PKB is not high
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
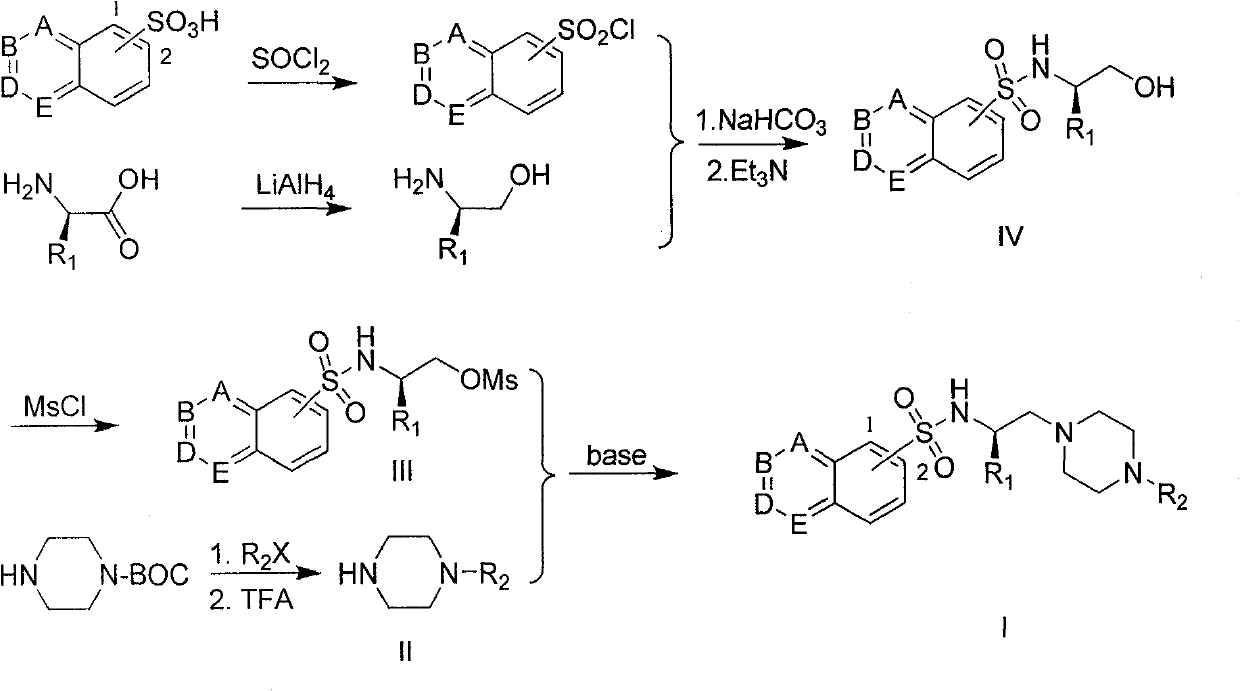
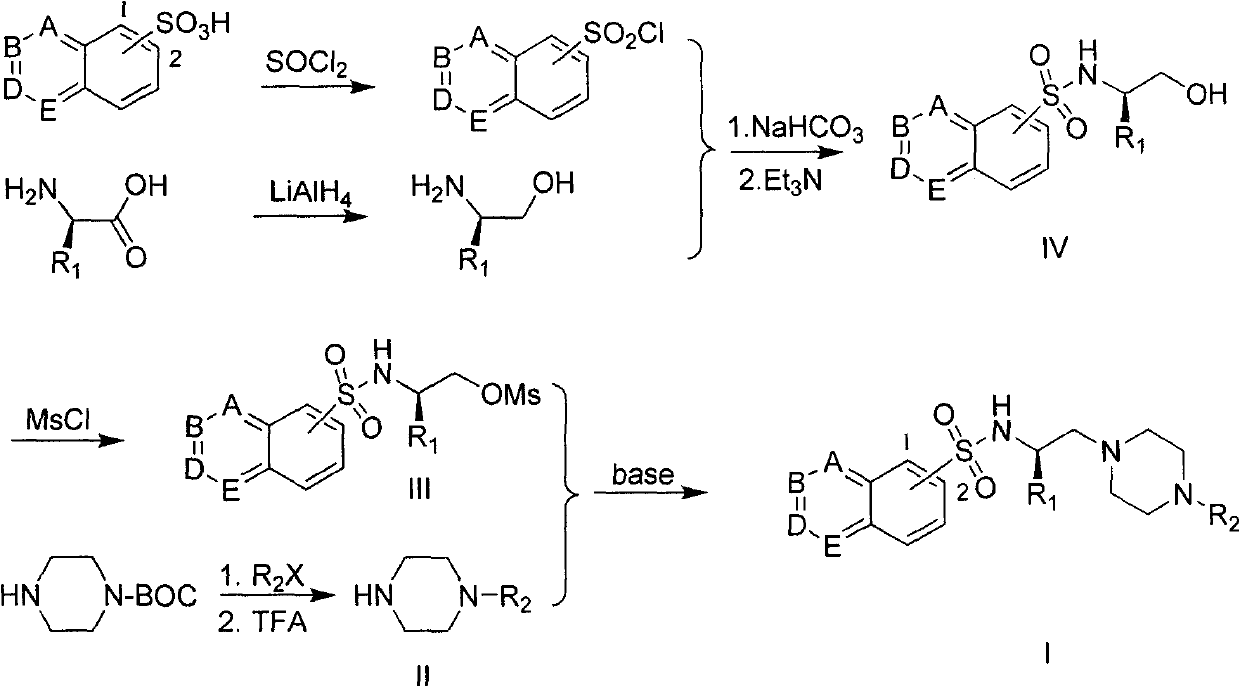
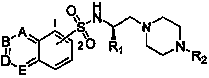
The invention provides a substituted piperazine N-ethyl sulfonamide derivative and medicinal salt thereof. The acyl chloride derivative thereof is obtained by chlorination of substituted sulfoacid by thionyl chloride; substituted L-type amino acid is reduced by lithium aluminium hydride reduction to obtain L-type alkamines compound; the L-type alkamines compound and the substituted sulfoacid derivative are carried out with coupling reaction and protected by mesyl chloride to obtain methanesulfonate derivative; mono-Boc protection piperazine is performed with substitution reaction and trifluoroacetic acid deprotection to obtain monosubstitution piperazine; and the monosubstitution piperazine is carried out with substitution reaction under the catalysis of organic amine to obtain the substituted piperazine N-Ethyl sulfonamide derivative. The virus activity test to five tumour cells in vitro by the compound provided by the invention shows that the in vitro virus activity test of the derivative shows the activity of parts of compound is higher than or comparative to positive control antitumor drug. The invention can be applied in preparing drug for preventing and curing tumour. The general formula of the compound of the invention is shown in the specification.
Description
field of invention The invention belongs to the field of medicinal chemistry and pharmacology, and relates to substituted piperazine ethyl sulfonamide derivatives and their preparation, as well as the application of the compounds in the preparation of antitumor drugs. Background of the invention Apoptosis is an important life phenomenon, and the abnormality of apoptosis is an important link in the occurrence and development of tumors. As an important molecule that mediates cell survival signals, protein kinase PKB plays an important role in the regulation of cell apoptosis. The activation process of PKB is the central part of the cell survival signal transduction pathway, and inhibiting the activity of PKB in various ways can promote the process of cell apoptosis. Protein kinase PKB will be a new and good antitumor drug target. In the current research on protein kinase inhibitors, Novartis's Gleevec and Astrazenca's Iressa have entered clinical application as drugs for the...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D217/02C07D417/12C07D401/12C07D295/13C07D277/32C07D213/61A61K31/496A61K31/495A61P35/00
Inventor 邹宏斌贾平祝华健章国林俞永平
Owner ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com