Strictosidine lactam derivatives and preparation method and use thereof
A technology of glycoside lactams, isoxaudioside, applied in the fields of enzymatic chemistry, medicinal chemistry and pharmacology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
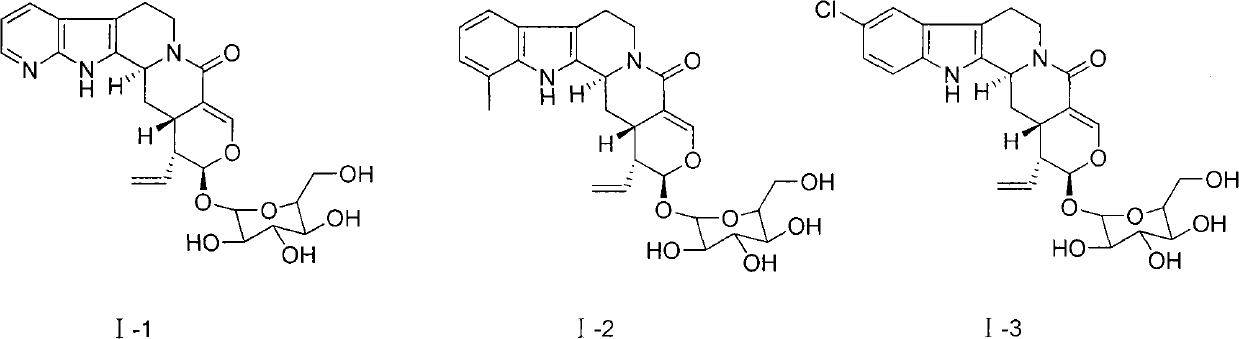
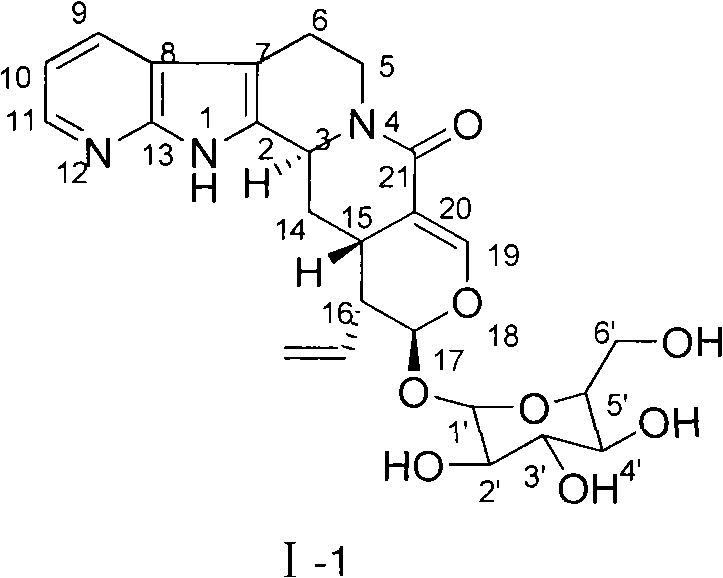
[0023] Example 1 :(3α,15β,16α,17β)-12-aza-19,20-didehydro-16-vinyl-isosorbinolactam-17-β-D-glucoside (compound I-1) preparation
[0024]
[0025] This example relates to a general synthesis method of a class of novel isoflavin lactam derivatives with cytotoxic activity as shown in formula (I). Specifically, it relates to the synthesis of (3α, 15β, 16α, 17β)-12-aza-19,20-didehydro-16-vinyl-isocurzin lactam-17-β-D-glucoside:
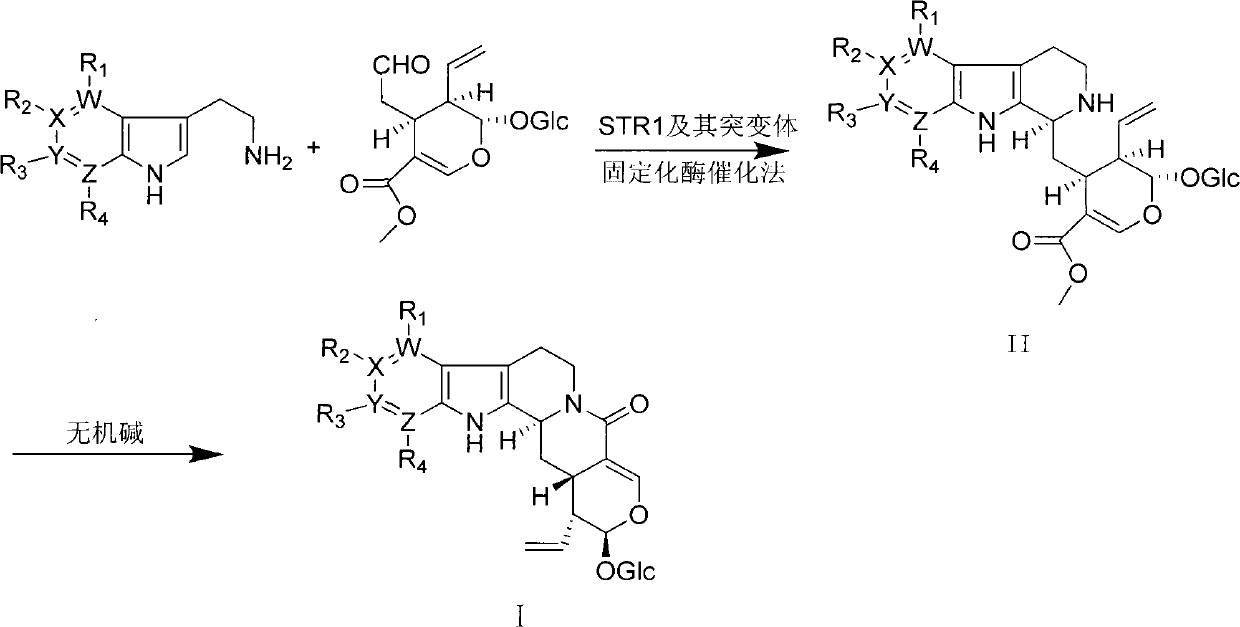
[0026] Dissolve 10 mg isoflavin synthase in phosphate buffer (50 mM, pH=7.0) at room temperature, and fix it on a Ni-NTA column. Take 1.61 g of 7-azatryptamine (10 mmol) and dissolve it in 100 mL of phosphate buffer (50 mM, pH=7.0). Then 3.88 g of serocycline (10 mmol) was dissolved in 100 mL of phosphate buffer (50 mM, pH=7.0). The mixture of the two was passed through a Ni-NTA column, the resulting effluent was cooled with liquid nitrogen and then freeze-dried, washed with methanol to remove the salt, and then prepared for liquid phase separation to obtain...
Embodiment 2
[0032] Example 2 : Preparation of Compound I-2 and I-3
[0033] According to the method of Example 1, using 7-methyltryptamine and 5-chlorotryptamine as raw materials, they are catalyzed by isofumarin synthase and mutants, and coupled with schiscycline to form the corresponding 12-methyl isocyanide. Visidin and 10-chloroisobridin are used as raw materials, and compounds I-2 and I-3 are prepared after being catalyzed by potassium carbonate and lithium carbonate, respectively.
[0034] Compound I-2: light yellow solid; yield 56.1%; Rf (chloroform / methanol 4:1) 0.56; ESI-MS m / z[M+H] + 513; 1 H NMR (500MHz, CD 3 OD): δ 7.65 (1H, d, J=8.0 Hz, H-9), 7.45 (1H, d, J=8.0 Hz, H-11), 7.37 (1H, s, H-19), 7.15 ( 1H, dd, J=8.0, 7.2 Hz, H-10), 5.64 (1H, m, CH 2 CH-16), 5.39 (1H, d, J = 2.1 Hz, H-17), 5.37 (1H, dd, J = 16.8, 1.4 Hz, CH 2 CH-16E), 5.32(1H, dd, J=10.5, 2.1Hz, CH 2 CH-16Z), 5.10 (1H, m, H-3), 4.93 (1H, dd, J=12.6, 5.6 Hz, H-5b), 4.56 (1H, d, J=8.4 Hz, H-1') , 3.85(1H, dd, J=11.9, 2...
Embodiment 3
[0037] Example 3: Cytotoxic activity of compound I-1 on A549 cells
[0038] A549 (human lung cancer) cells were cultured with RPMI 1640 medium containing 10% fetal bovine serum, 100 U / ml penicillin and 100 U / ml streptomycin. 5×10 cells per well 3 Added to 96-well plate with 5% CO at 37℃ 2 Incubate for 24 hours in a humid air incubator.
[0039] The cell survival rate was determined by the modified MTT method. After the cells were incubated for 24 hours, the newly prepared dimethyl sulfoxide solution of compound I-1 was added to each well in a concentration gradient, so that the final concentration of the compound in the well was 100 μg / ml and 33.3 μg / ml, respectively. 11.1 μg / ml and 3.7 μg / ml. After 72 hours, add 10 microliters of MTT (5 mg / ml) phosphate buffer, then continue to incubate at 37°C for 4 hours, centrifuge for 5 minutes to remove unconverted MTT, and add 200 microliters of dimethylsulfoxide to each well Sulfone is used to dissolve the reduced MTT crystals formazan,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com