Compositions for treating tumor diseases
A composition and disease technology, applied in the field of compositions for the treatment of tumor diseases, can solve the problems of less serious hematological toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
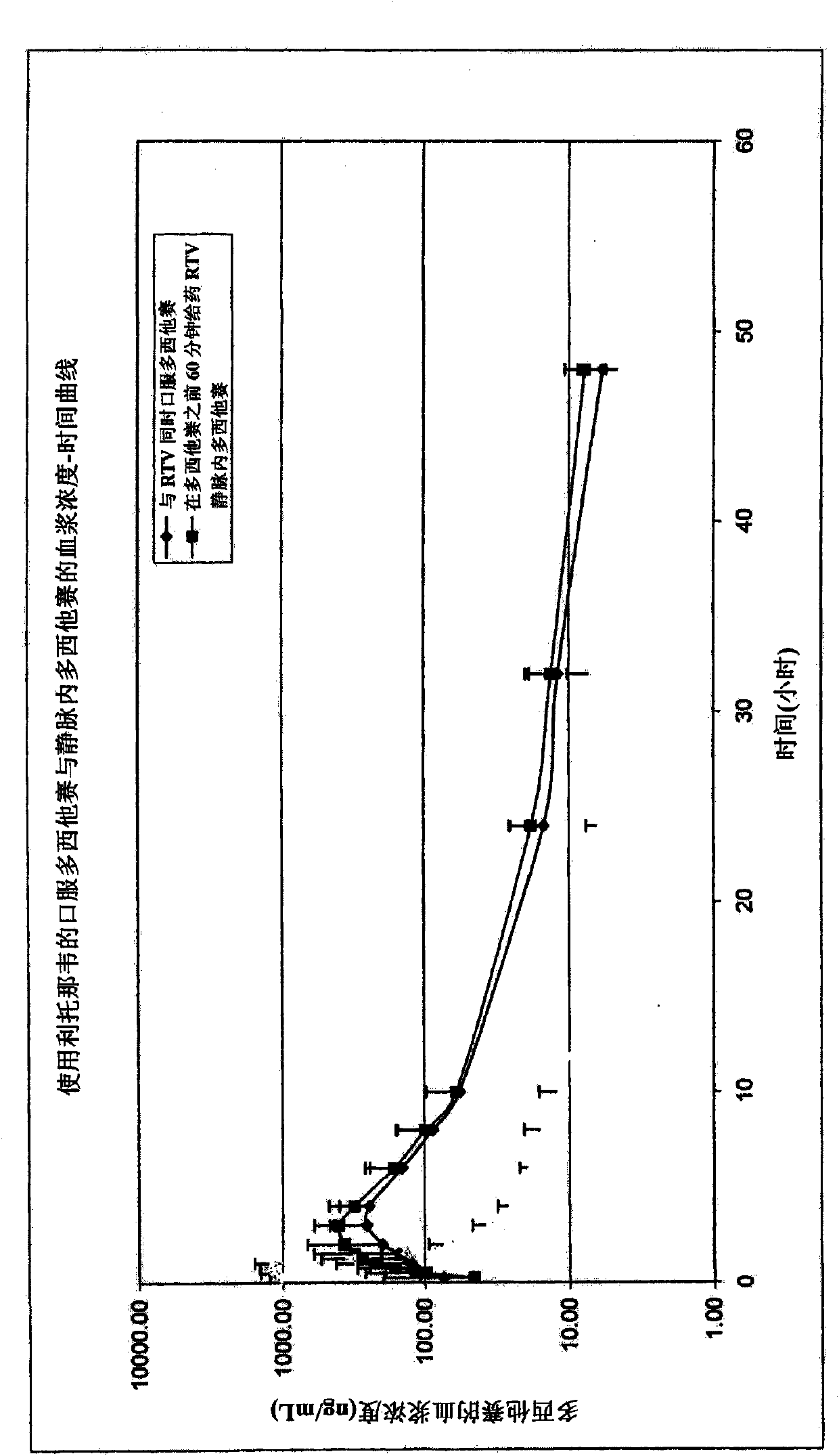
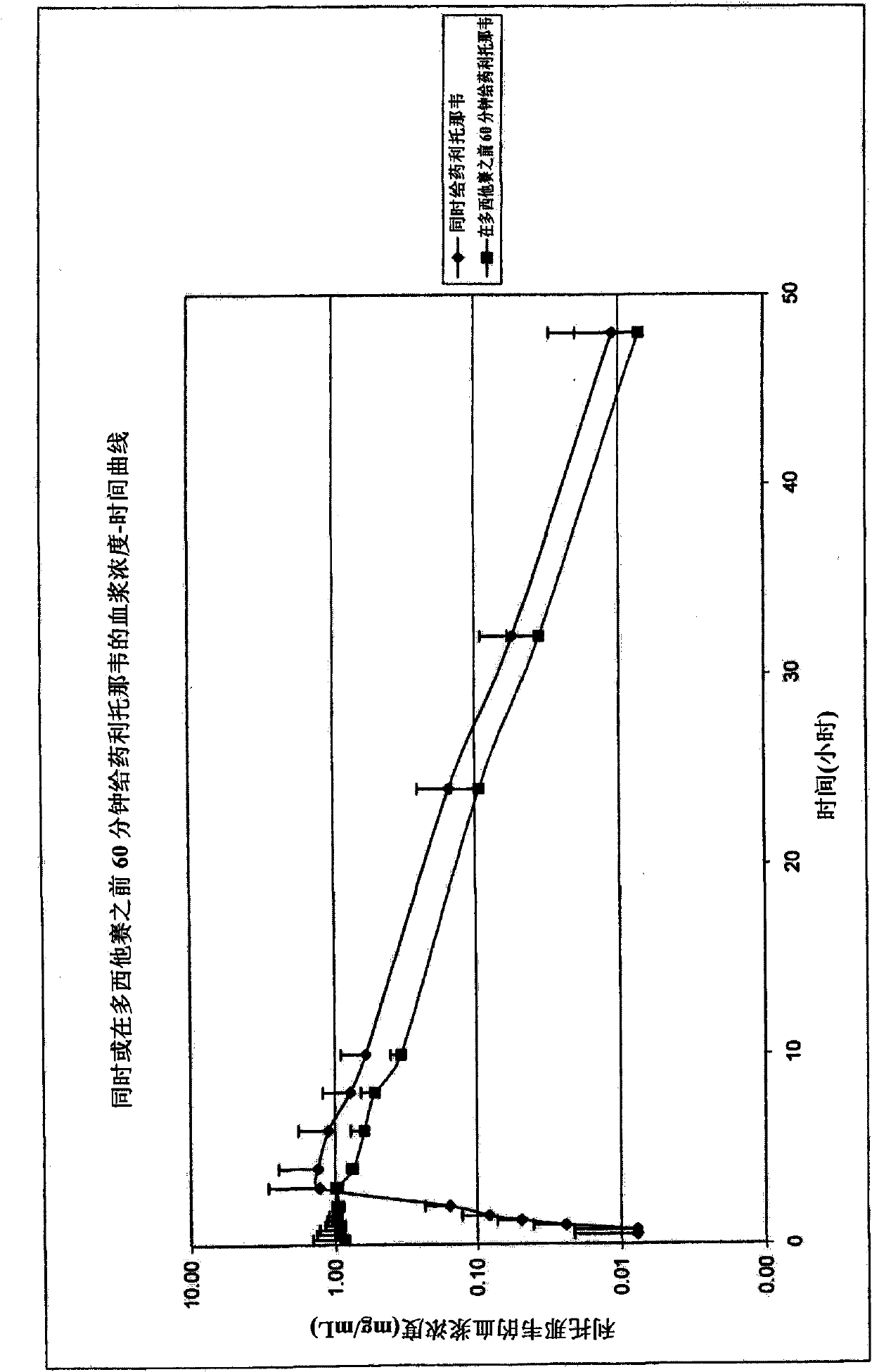
[0134] A 100 mg ritonavir dose was combined with a 100 mg docetaxel dose and administered orally to 22 patients simultaneously. with intravenous docetaxel (100mg) In comparison, dosing was administered as a 1-hour intravenous infusion (standard method) (without ritonavir).
[0135] Oral ritonavir: 1 capsule contains 100 mg ritonavir . Oral docetaxel dose: 100mg. A commercially available intravenous docetaxel formulation ( 2ml=80mg docetaxel; excipient polysorbate 80) is diluted with ethanol 95%: water (13:87), provides 10mg / ml docetaxel solution, let patient drink together with 100ml tap water (10ml 10mg / ml solution).
[0136] The obtained pharmacodynamic data are as follows:
[0137] Without ritonavir, the AUC of docetaxel administered orally is 0.29±0.26(mg.h / L)
[0138] With ritonavir, the AUC of docetaxel administered orally was 2.4±1.5(mg.h / L)
[0139] Without ritonavir, AUC of intravenous docetaxel 1.9±0.4(mg.h / L)
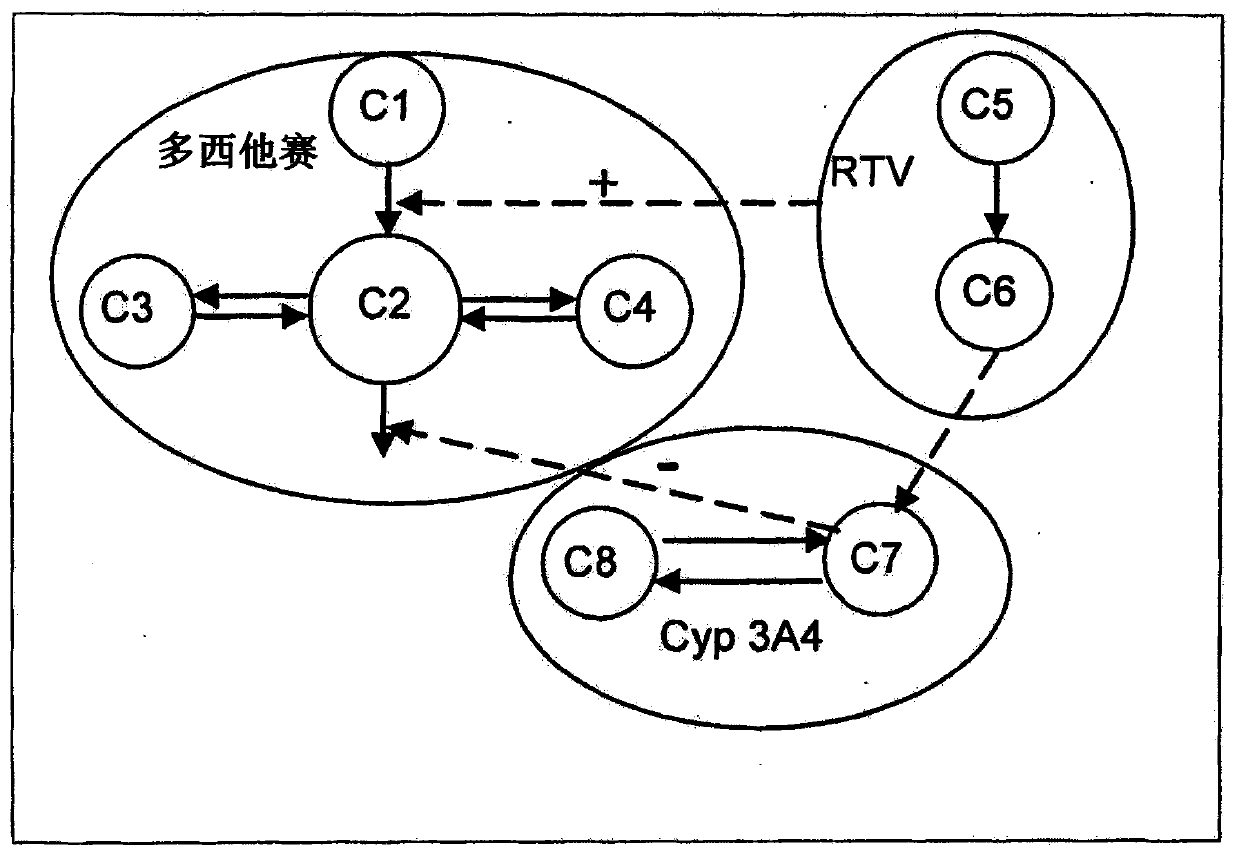
[0140] The results indicated a dual effect ...
Embodiment 2
[0144] Oral combination of docetaxel and ritonavir for the treatment of solid malignancies.
[0145] Patients are randomized into two treatment groups, X and Y. Group X, in the first week, received 100 mg ritonavir, 60 minutes later, received 100 mg oral docetaxel, and in the second week, these patients received both 100 mg ritonavir and 100 mg oral docetaxel. Patients in group Y received 100 mg ritonavir and 100 mg oral docetaxel concurrently during the first week, and 100 mg ritonavir followed 60 minutes later by 100 mg oral docetaxel during the second week. 15 days after the start of oral administration, both X and Y groups received 100 mg of intravenous docetaxel without ritonavir ( Standard method; 1 hour perfusion).
[0146] Oral docetaxel dose: 100mg. A commercially available intravenous docetaxel formulation ( 2ml=80mg docetaxel; excipient polysorbate 80) is diluted with ethanol 95%: water (13:87), provides 10mg / ml docetaxel solution, let patient drink together w...
Embodiment 3
[0181] Oral formulation of embodiment 3-paclitaxel
[0182] 3.1: Solid Dispersions Compared to Physical Mixtures
[0183] In this experiment, the solubility and dissolution rate of a composition comprising a solid dispersion of paclitaxel and PVP-K17 mixed with SDS was compared to a physical mixture of anhydrous paclitaxel, PVP-K17 and SDS.
[0184] Paclitaxel solid dispersion in PVP-K 17 5 mg capsules
[0185] A 20% solid dispersion of paclitaxel in PVP-K17 was prepared by dissolving 100 mg of paclitaxel in 10 mL of t-butanol and 400 mg of PVP-K17 in 6.67 mL of water. With continuous stirring, the paclitaxel / tert-butanol solution was added to the PVP-K17 / water solution. The final mixture was transferred to an 8 mL vial with a maximum fill level of 2 mL. The tert-butanol and water were subsequently removed by lyophilization (see Table 3 for conditions). 25 mg paclitaxel 20% / PVP-K17 solid dispersion (=5 mg paclitaxel) was mixed with 125 mg lactose, 30 mg sodium lauryl sulfa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| crystallinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com