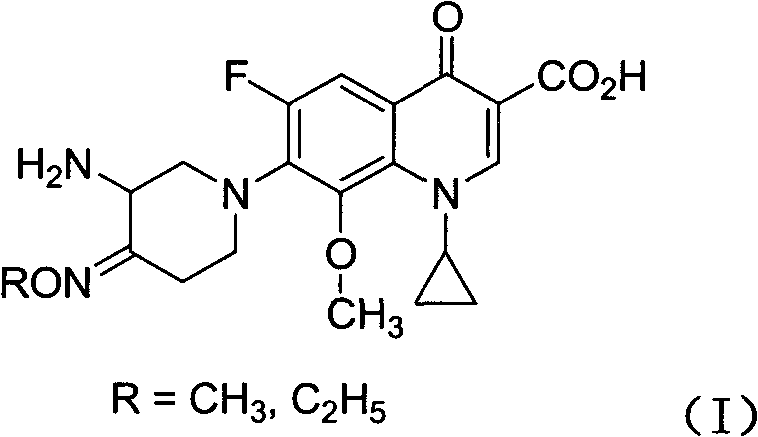
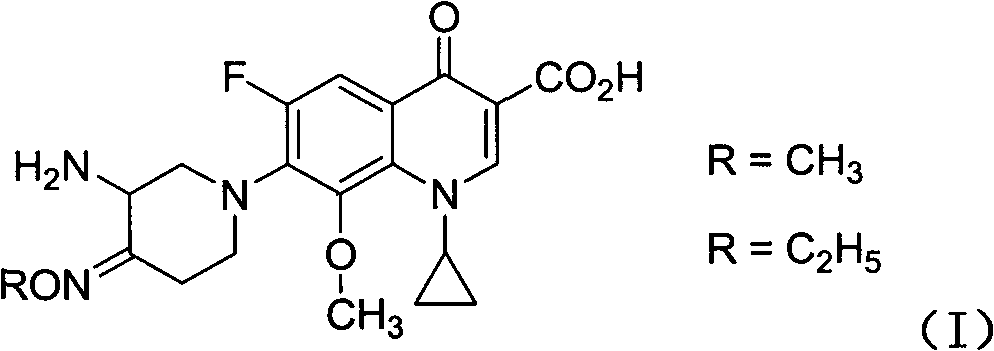
Fluoroquinolone comprising 7-(3-amino-4-oximido)-1-piperidyl substitutional group and application of composition thereof
A fluoroquinolone, piperidinyl technology, applied to fluoroquinolone compounds, the application field in the treatment of tuberculosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
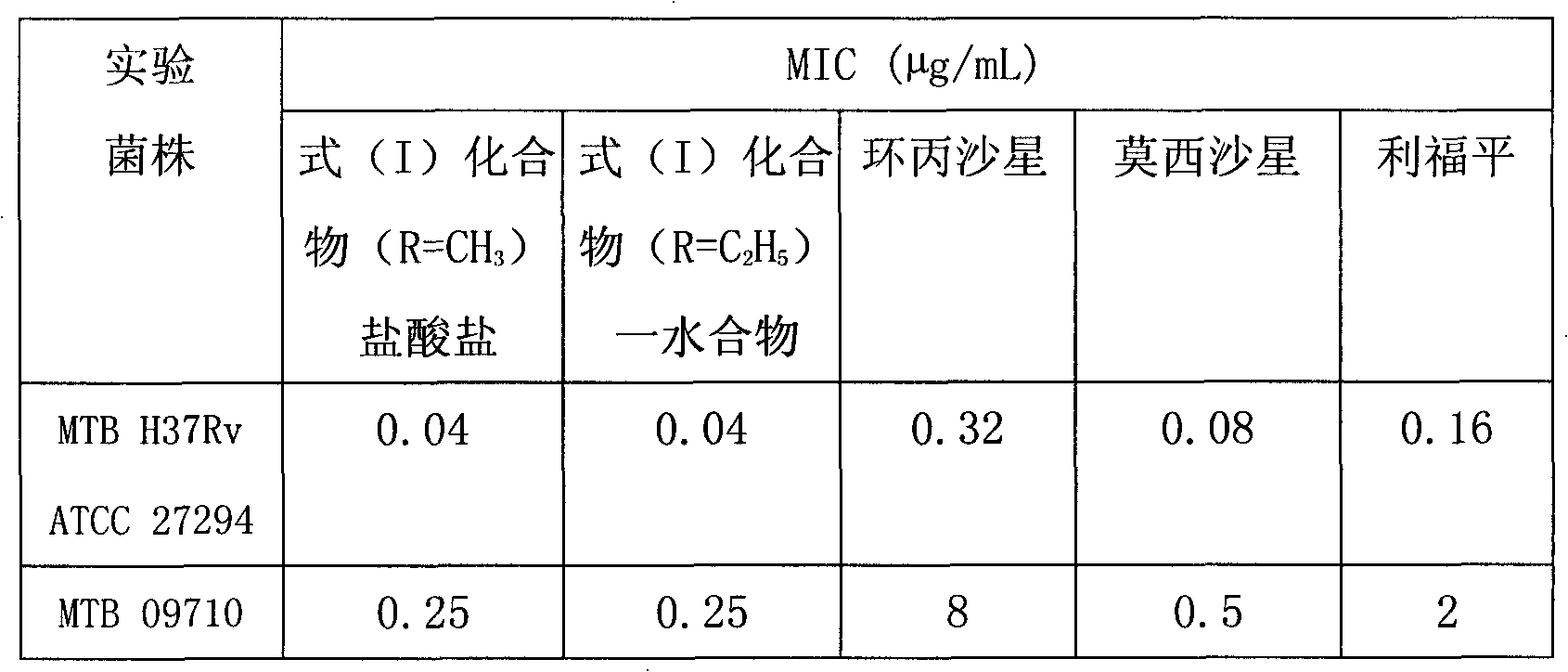
[0018] In vitro anti-mycobacterial activity test
[0019] The anti-tuberculosis activity of the compound shown in formula (I) or its medicinal salt, hydrate is by measuring its minimum inhibitory concentration (MIC, μ g / mL) to Mycobacterium tuberculosis standard strain H37Rv ATCC 27294 and clinical isolate 09710 ) to represent. In this trial, ciprofloxacin and moxifloxacin and the first-line anti-tuberculosis drug rifampicin were used as control drugs. The minimum inhibitory concentration was determined as follows: sterile 48-well plate (special microculture plate for rapid drug susceptibility of Mycobacterium tuberculosis), according to the design requirements of the drug susceptibility test, each well was added with 2 times concentration medium (improved Michaelis 7H9 liquid culture medium) base) diluted drug. Each compound was made into an initial solution of appropriate concentration, diluted with culture medium (2×) to double the concentration of each compound used, eac...
Embodiment 2
[0025] Oral acute toxicity test
[0026] In order to measure the oral acute toxicity of the compound represented by the formula (I) or its pharmaceutically acceptable salt, hydrate, it has been carried out acute toxicity test, will contain the compound represented by the formula (I) or its pharmaceutically acceptable salt of different concentrations, The solution of hydrate was orally given to male mice, and the dose was 0.1ml / 10g body weight. After 7 days, the amount of dead mice was counted respectively, and the median lethal dose (LD) of each compound was calculated by Bliss program. 50 ). The results are listed in Table 2.
[0027] Oral acute toxicity in mice of table 2 experimental compounds
[0028] experimental compound
LD 50 (mg / kg)
Formula (I) compound (R=CH 3 )
>4000
Formula (I) compound (R=CH 3 )Hydrochloride
>4000
Formula (I) compound (R=C 2 h 5 ) monohydrate
>4000
[0029] Experimental result...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com