Preparation method of alpha crystalline azelnidipine
A technology of azedipine and its crystal form, which is applied in the field of preparation of medicinal compounds, can solve the problems of restricting industrial use, and achieve the effects of low toxicity, high purity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
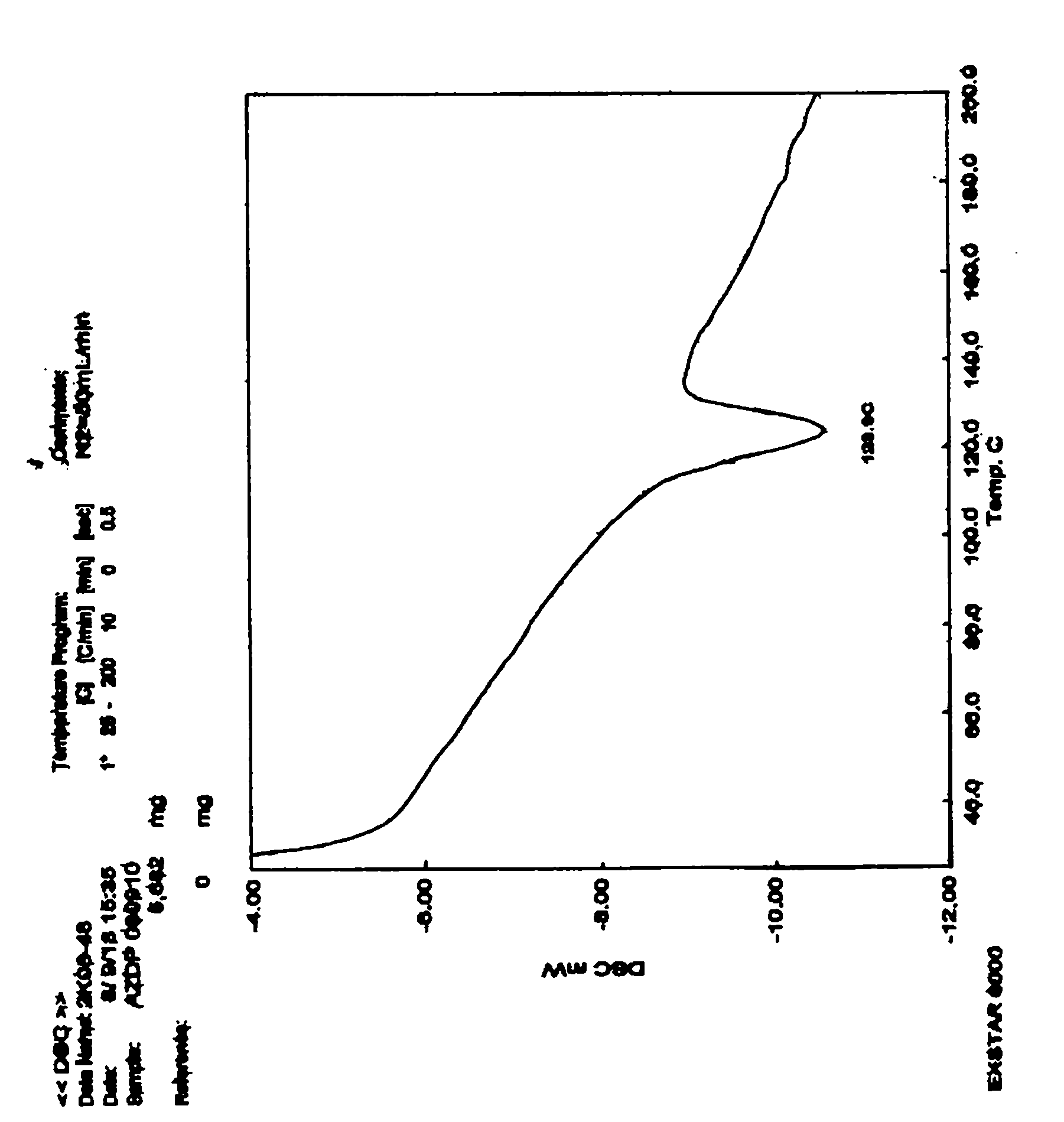
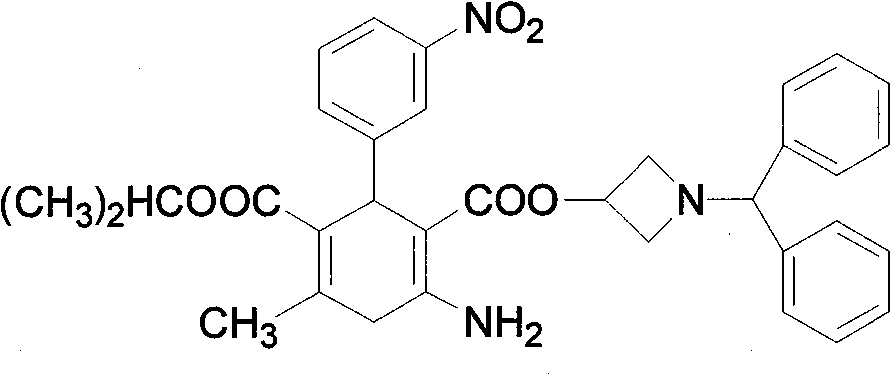
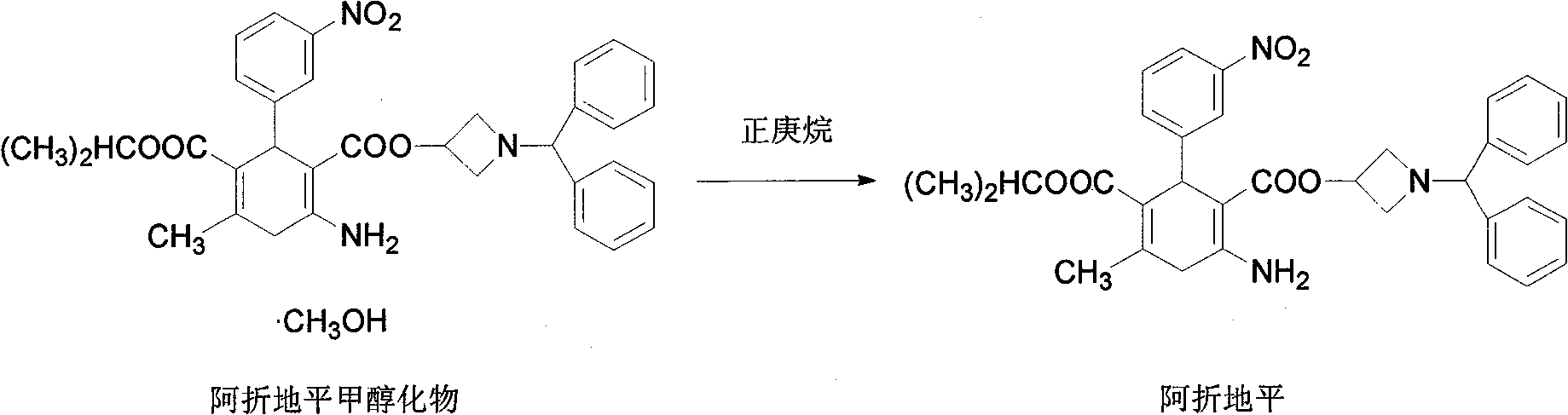
[0024] Prepare Azedipine methanolate according to the conventional method; Add 200g of Azedipine methanolate into a 5000ml reaction bottle, then add 4000ml of n-heptane, stir evenly and then heat up, the temperature is 45 under the pressure condition of -0.3~-0.095Mpa At -50°C, the solvent was evaporated by distillation under reduced pressure. When the distillation fraction reached about 800ml, the distillation was stopped, stirred and cooled to crystallize, filtered with suction, washed with n-heptane, and dried in vacuum at 60°C to obtain 189g of azedipine with a yield of 94.5%. DSC: 123.9°C (absorption peak), pharmaceutical α crystal form, HPLC content>99.5% (area normalization method, single impurity<0.1%), residual solvents meet the requirements.
Embodiment 2
[0026] Prepare Azedipine methanolate according to a conventional method; the content of methanol in the prepared Azedipine methanolate is about 4-9%; add 200 g of Azedipine methanolate to a 5000ml reaction flask, then add 2000ml of n-heptane, After stirring evenly, heat up and heat up. When the temperature reaches 55-60°C under the pressure condition of -0.3~-0.095Mpa, the solvent is evaporated by distillation under reduced pressure. When the distilled fraction reaches about 800ml, stop the distillation, stir and cool to crystallize, filter with suction, wash with n-heptane, and dry in vacuum at 50°C to obtain 192g of azelnidipine with a yield of 96.0%. DSC: 123.8°C (absorption peak), pharmaceutical α crystal form, HPLC content>99.5% (area normalization method, single impurity<0.1%), residual solvents meet the requirements.
Embodiment 3
[0028] Azedipine methanolate is prepared according to the conventional method; the content of methanol in the prepared Azedipine methanolate is about 4-9%; add 200 g of Azedipine methanolate to 6000 ml of n-heptane, stir evenly and then heat up and heat up at - When the temperature reaches 50-55°C under the pressure condition of 0.3~-0.095Mpa, the solvent is evaporated by distillation under reduced pressure. When the distillation fraction reaches about 1000ml, stop the distillation, stir and cool to crystallize, filter with suction, wash with n-heptane, and dry under vacuum at 50°C to obtain 188g of azedipine with a yield of 94.0%. DSC: 123.7°C (absorption peak), pharmaceutical α crystal form, HPLC content>99.5% (area normalization method, single impurity<0.1%), residual solvents meet the requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com