Preparation method of azelnidipine alpha crystal form
A technology of azeldipine and crystal form, which is applied in the field of raw material drug preparation, can solve the problems of high production cost, mixed crystals, difficult solvent selection, etc., and achieves the effects of convenient crystal transformation, stable quality and simple process operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of Azedipine Diisopropanolate and α Crystal Form
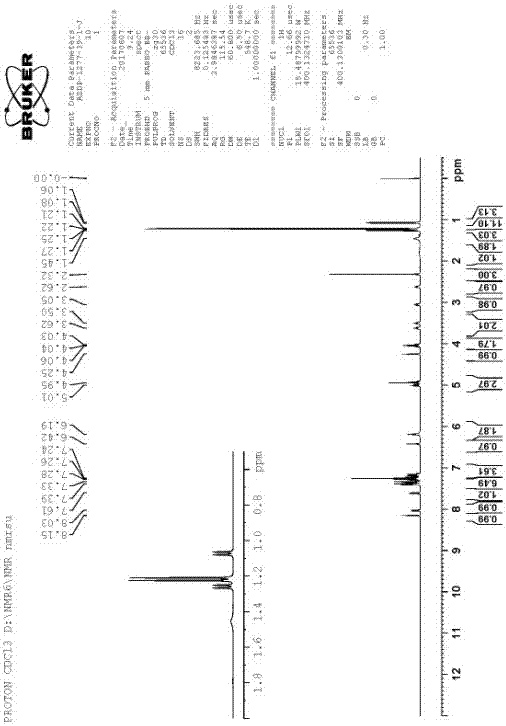
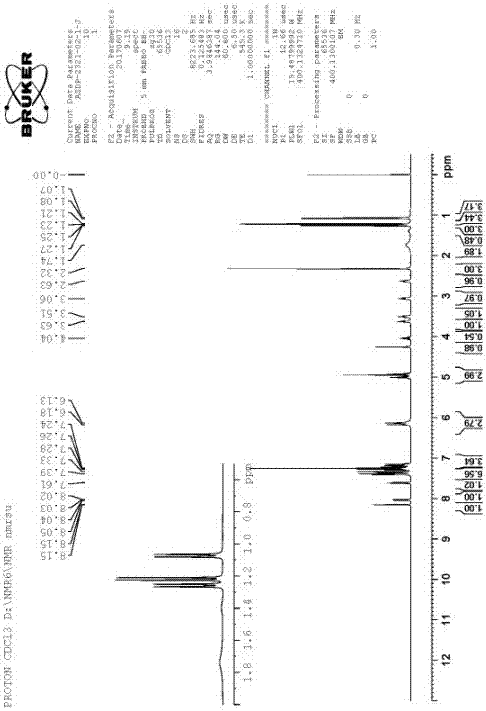
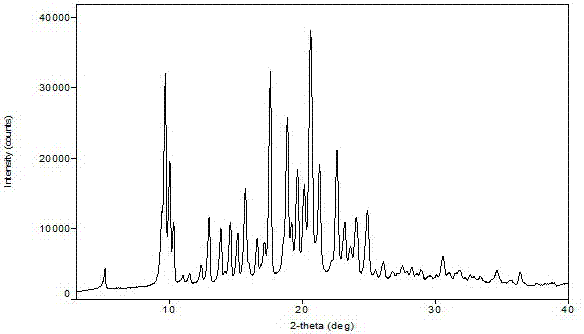
[0041] Add 500g of isopropanol to the crude product of Azedipine (converted to 100g of pure Azedipine), heat and reflux until it dissolves, cool down to 28°C to start crystallization, continue to cool down to -10°C and keep warm for 4 hours, and filter to obtain yellow The solid was dried at room temperature to obtain 108.9 g of azeldipine diisopropanolate. Yield 90.3%, HPLC purity 99.91%. As determined by gas phase, the content of isopropanol was 16.8% (theoretical amount was 17.1%). Its nuclear magnetic spectrum, crystal form determination and DSC are shown in the attached drawings (2, 4, 6)
[0042] Add 50 g of the obtained azelnidipine diisopropanolate to 300 g of cyclohexane, heat and reflux for 3 hours, cool down to 10-20°C and continue to keep warm for 2 hours, filter, and vacuum dry to obtain 39.2 g of yellow powder, yield 94.6% , HPLC purity 99.97%. . Its crystal form was determined t...
Embodiment 2
[0043] Example 2 Preparation of Azeldipine Diisopropanolate and α Crystal Form
[0044] Add 100g of Azedipine in the β crystal form into 1000g of isopropanol, heat to reflux to dissolve, cool down to 32°C, crystallization begins, continue to cool down to -10°C and keep warm for 4 hours, filter to obtain a yellow solid, and let it dry at room temperature After drying, 99.6 g of diisopropanolate was obtained, the yield was 82.6%, and the HPLC purity was 99.96%. The content of isopropanol was 17.3% (theoretical amount was 17.1%) as determined by gas phase. Its crystal form determination is consistent with Example 1.
[0045] Add 50 g of the obtained azelnidipine diisopropanolate to 400 g of cyclohexane, lower the temperature to 10-15 ° C and keep stirring for 6 hours, filter, and vacuum-dry to obtain 38.4 g of yellow powder, the yield is 92.6%, and the HPLC purity is 99.98% . The determination of its crystal form is completely consistent with Example 1.
example 3
[0046] Example 3 Preparation of Azeldipine Diisopropanolate and α Crystal Form
[0047] Add 100g of amorphous azedipine into 800g of isopropanol, heat to reflux to dissolve, cool down to 35°C, crystallization begins, continue to cool down to -10°C and keep warm for 4 hours, filter to obtain a yellow solid, and dry at room temperature , 104.8 g of diisopropanolate was obtained, the yield was 86.9%, and the HPLC purity was 99.93%. The content of isopropanol was 18.1% (theoretical amount was 17.1%) as determined by gas phase. Its crystal form determination is consistent with Example 1.
[0048] Add 50 g of the obtained azelnidipine diisopropanolate to 1000 g of cyclohexane, raise the temperature to 40-45°C and keep stirring for 4 hours, filter, and vacuum-dry to obtain 36.8 g of yellow powder with a yield of 88.8% and an HPLC purity of 100.0%. The determination of its crystal form is completely consistent with Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com