A kind of preparation method of azedipine impurity b
A technology for azeldipine and impurities, which is applied in the field of raw material drug preparation and can solve the problems of large solvent ratio, long reaction time, low conversion rate and content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
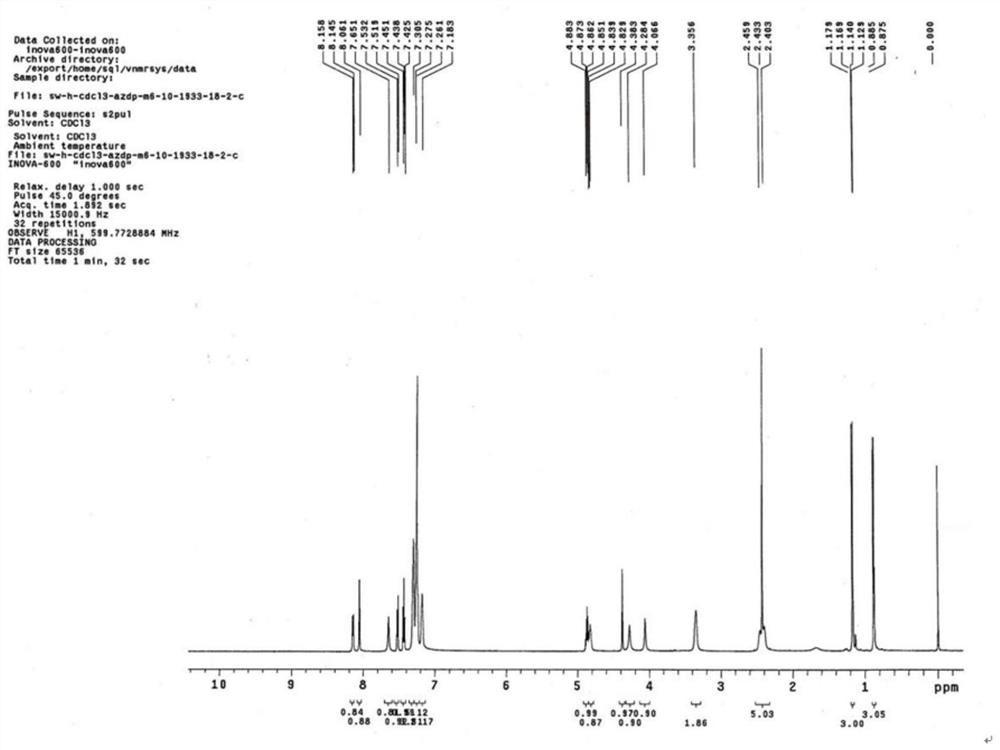
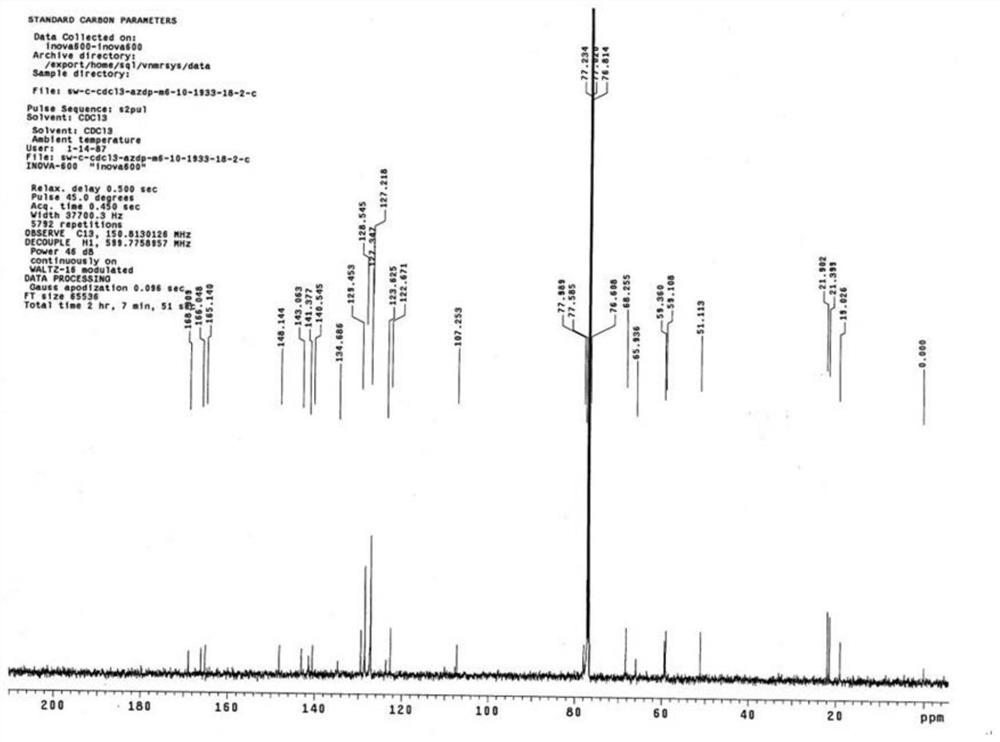
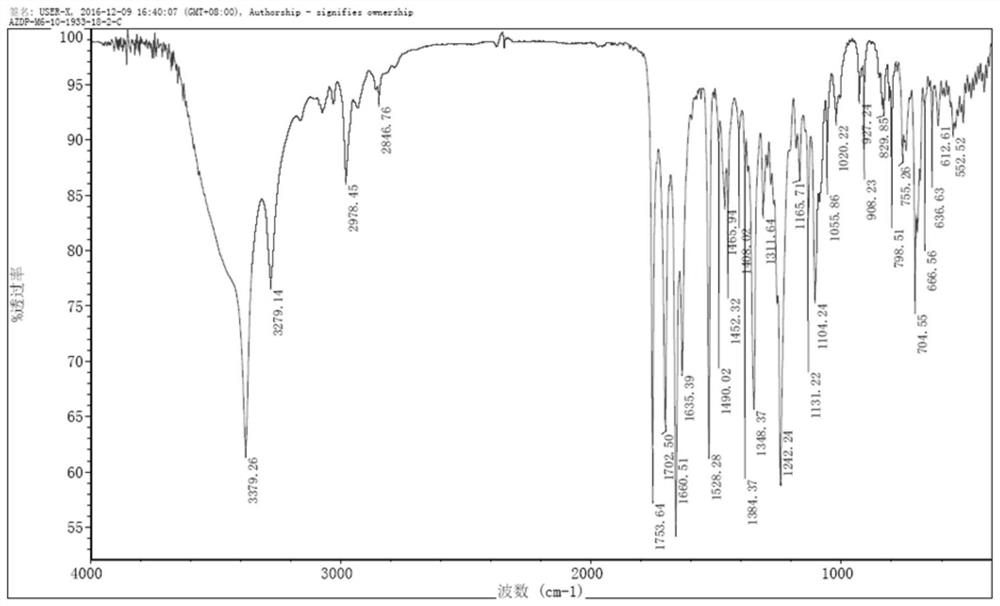
[0026] Add 2.0 g (3.4 mmol) of azelnidipine and 30 ml of dichloromethane into a 100 ml three-neck flask, dissolve and cool to -15°C. Add 0.6g (3.5mmol) m-chloroperoxybenzoic acid (dissolved in 10ml of dichloromethane) dropwise to the reaction system, keep the dropwise addition process at -10°C, add it in about 20 minutes, and react at -15°C for 10 minutes , TLC monitors that the reaction is complete, moves to room temperature, adds 30ml of saturated aqueous solution of sodium bisulfite and stirs for 20min, separates to take the dichloromethane phase, adds 20ml of saturated sodium bicarbonate and washes twice, retains the organic phase, and decomposes at 30-35°C. The dichloromethane solvent was removed by autoclaving to obtain a viscous oil.
[0027] Put the above viscous oil into a chromatographic column containing 60g of chromatographic silica gel, use dichloromethane:methanol=20:1 as the eluent, collect the part with ultraviolet absorption, concentrate, crystallize, filter, ...
Embodiment 2
[0039] Add 10.0 g (17.2 mmol) of azelnidipine and 100 ml of dichloromethane into a 500 ml three-neck flask, dissolve it, and cool down to -10°C. Add 3.0g (17.4mmol) m-chloroperoxybenzoic acid (dissolved in 50ml of dichloromethane) dropwise into the reaction system, keep the dropwise addition process at -10°C, add about 30min, and react at -10°C for 10min , TLC monitors that the reaction is complete, move to room temperature, add 200ml of saturated aqueous solution of sodium bisulfite and stir for 20min, separate layers to take the dichloromethane phase, add 200ml of saturated sodium bicarbonate to wash twice, keep the organic phase, and reduce The dichloromethane solvent was removed by autoclaving to obtain a viscous oil.
[0040] Put the above viscous oil into a chromatographic column containing 200g of chromatographic silica gel, use dichloromethane:methanol=20:1 as the eluent, collect the part with ultraviolet absorption, concentrate, crystallize, filter, Dried at 50°C to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com