Azelnidipine crystal form, preparation method for same and officinal composition thereof
A technology of Azhedipine and crystal form, which is applied in the field of medicine, can solve the problems of unfavorable production operation and high static electricity of Azhedipine α crystal form, and achieve the effect of small electrostatic interaction, simple production operation and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
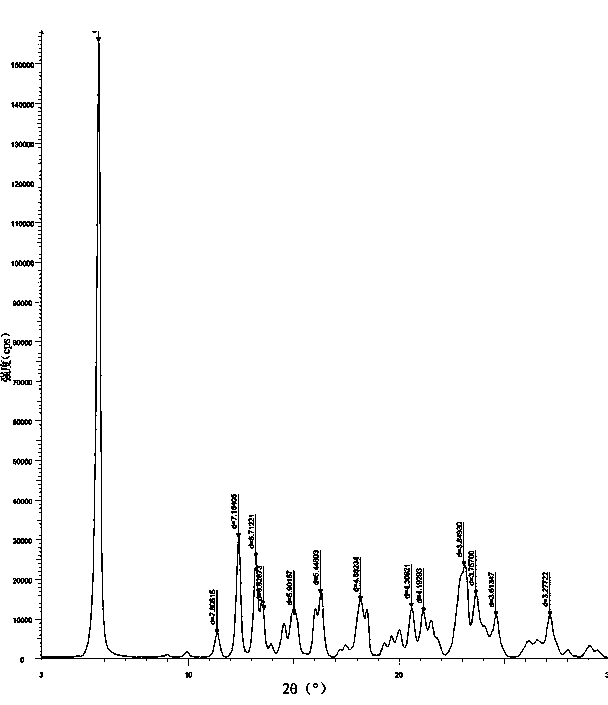
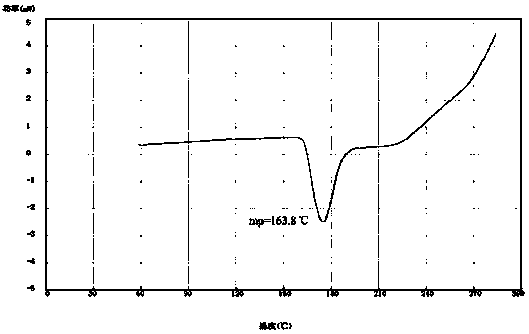
[0023] Thoroughly mix 10 g of amorphous azedipine with 40 ml of toluene, and heat to 30-40°C. Then add 2 to 3 g of activated carbon, stir and filter, add 40 ml of cyclohexane to the filtrate while stirring, continue to stir and crystallize, filter and dry to obtain 9.5 g of the new crystal form of azedipine, with a yield of 95% and a melting point of 163.4°C.
[0024] Heating to 40-50°C, and performing the same operations as above to obtain 9.4 g of the new crystal form of azelnidipine, with a yield of 94% and a melting point of 162.1°C.
[0025] Heating to 50-60°C, and performing the same operations as above to obtain 9.4 g of the new crystal form of azelnidipine, with a yield of 94% and a melting point of 162.7°C.
[0026] The above test proves that the heating and dissolving temperature ranges from 30 to 60°C. The higher the heating temperature, the longer the required crystallization time, so the preferred temperature is 30-40°C.
Embodiment 2
[0028] Thoroughly mix 10 g of amorphous azedipine with 40 ml of toluene, and heat to 30-40°C. Then add 2 to 3 g of activated carbon, stir and filter, add 40 ml of cyclohexane to the filtrate while stirring, continue to stir and crystallize, filter and dry to obtain 9.5 g of the new crystal form of azedipine, with a yield of 95% and a melting point of 163.8°C.
[0029] Using 50ml of toluene and 50ml of cyclohexane each, the rest was the same as above to obtain 9.4g of the new crystal form of azelnidipine, with a yield of 94% and a melting point of 163.7°C.
[0030] Using 60ml each of toluene and cyclohexane, the rest was the same as above to obtain 9.3g of the new crystal form of azeldipine, with a yield of 93% and a melting point of 162.2°C.
[0031] The above experimental results show that: for 10 g of amorphous azedipine, the preferred dosage of toluene is 40-60 ml. Experimental studies have found that when 10g of azeldipine is mixed with 40-60ml of toluene, the concentrati...
Embodiment 3
[0033] Thoroughly mix 10 g of amorphous azedipine with 40 ml of toluene, and heat to 30-40°C. Then add 2 to 3 g of activated carbon, stir and filter, add 20 ml of cyclohexane to the filtrate while stirring, continue to stir and crystallize, filter and dry to obtain 8.9 g of the new crystal form of azedipine with a yield of 89% and a melting point of 164.1°C.
[0034] 40ml of cyclohexane was added, and the rest was performed as above to obtain 9.5g of the new crystal form of azeldipine, with a yield of 95% and a melting point of 163.1°C.
[0035] 80ml of cyclohexane was added, and the rest was the same as above to obtain 9.6g of the new crystal form of azeldipine, with a yield of 96% and a melting point of 162.9°C.
[0036] The above experimental results show that the volume ratio of cyclohexane to toluene is 1:2 to 2:1, preferably an equal volume ratio. Too little cyclohexane will cause the yield to decrease, and when it reaches 1:1, the new crystal form of azeldipine is almo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com