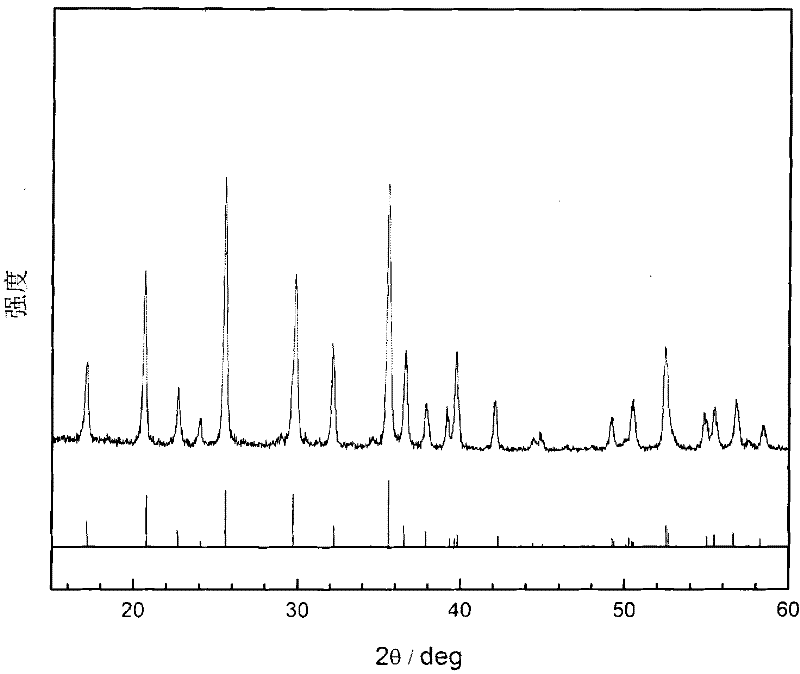
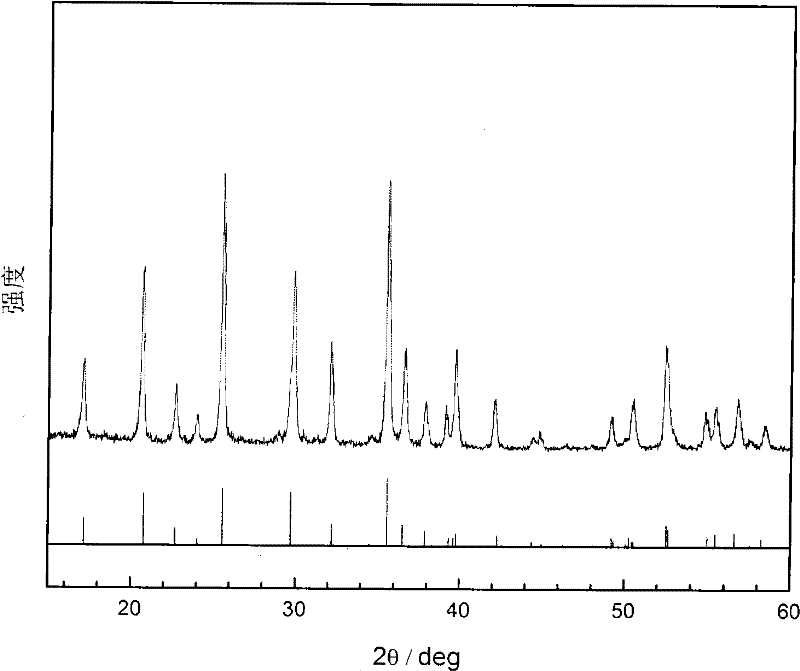
Preparation method of pure-phase high-crystallinity lithium iron phosphate
A technology with high crystallinity and lithium iron phosphate, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as low crystallinity, harsh conditions, and complicated processes, and achieve simple equipment, reduced energy consumption, and diffraction high intensity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Dissolve 0.02mol of ferrous sulfate in 10mL of water, then add 20mL of ethylene glycol to form a mixture of ferrous sulfate-water-alcohol, add it to a flask with a reflux device, stir it evenly, and set aside ;
[0027] (2) Dissolve 2g of sodium dodecylsulfonate in 10mL of water, and then add 20mL of ethylene glycol to form a mixture of sodium dodecylsulfonate-water-alcohol for later use;
[0028] (3) Dissolve 0.02mol of diammonium hydrogen phosphate in 10mL of water, then add 20mL of ethylene glycol to prepare a mixed solution of diammonium hydrogen phosphate-water-alcohol for use;
[0029] (4) Dissolve 0.02mol of lithium hydroxide in 10mL of water, then add 20mL of ethylene glycol to prepare a lithium hydroxide-water-alcohol mixture for use;
[0030] (5) Preheat the mixed solution prepared in steps (2), (3), and (4) to 60°C, quickly add them to the flask in step (1) successively, and raise the temperature to 118°C under nitrogen protection, Reflux reaction for 2...
Embodiment 2
[0036] The diammonium hydrogen phosphate in the step (3) in the embodiment 1 is replaced by the ammonium dihydrogen phosphate, and other steps are with embodiment 1. The obtained product was 2.95 g of pure-phase high-crystallinity lithium iron phosphate powder, the crystal state and structure of which were the same as in Example 1. The initial discharge capacity at 0.2C was 152.5 mAh / g.
Embodiment 3
[0038] The diammonium hydrogen phosphate in the step (3) in the embodiment 1 is replaced by phosphoric acid, and other steps are with embodiment 1. The obtained product was 2.97g of pure-phase high-crystallinity lithium iron phosphate powder, the crystal state and structure of which were the same as in Example 1. The initial discharge capacity at 0.2C was 152.8mAh / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com