Method for constructing autoimmune recurrent abortion mouse model
A technology for recurrent miscarriage and construction methods, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, antibody medical ingredients, etc., can solve the problems of lack of autoimmune RSA animal models, number of implanted embryos, embryo loss rate and ACA drop In order to achieve good application prospects, reduce modeling time, improve the success rate and efficiency of modeling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] 1. Materials and methods
[0010] 1.1 Reagents
[0011] Human β2GP-1 (β2-glycoprotein-1) was purchased from Cell Sciences, USA; CFA (Freund's Adjuvant Complete) and IFA (Freund's Adjuvant Incomplete) were purchased from Sigma, USA; BALB / c mice were purchased from China Provided by the Shanghai Experimental Animal Center of the Academy of Sciences.
[0012] 1.2 Experimental animals
[0013] 8-week-old clean-grade female and male BALB / c inbred mice, provided by the Shanghai Experimental Animal Center of the Chinese Academy of Sciences, weighing 20±2g.
[0014] 2. Construction of animal models
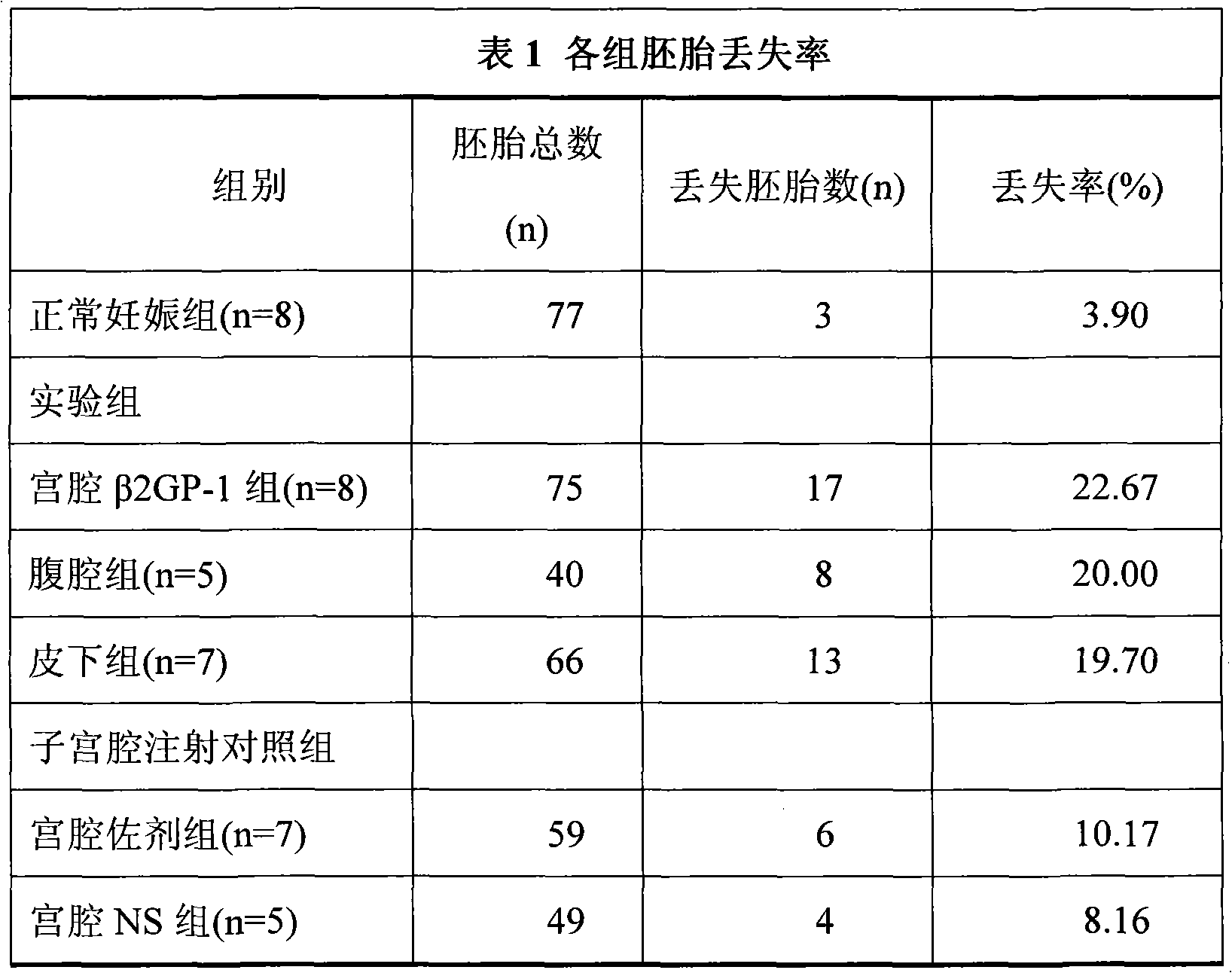
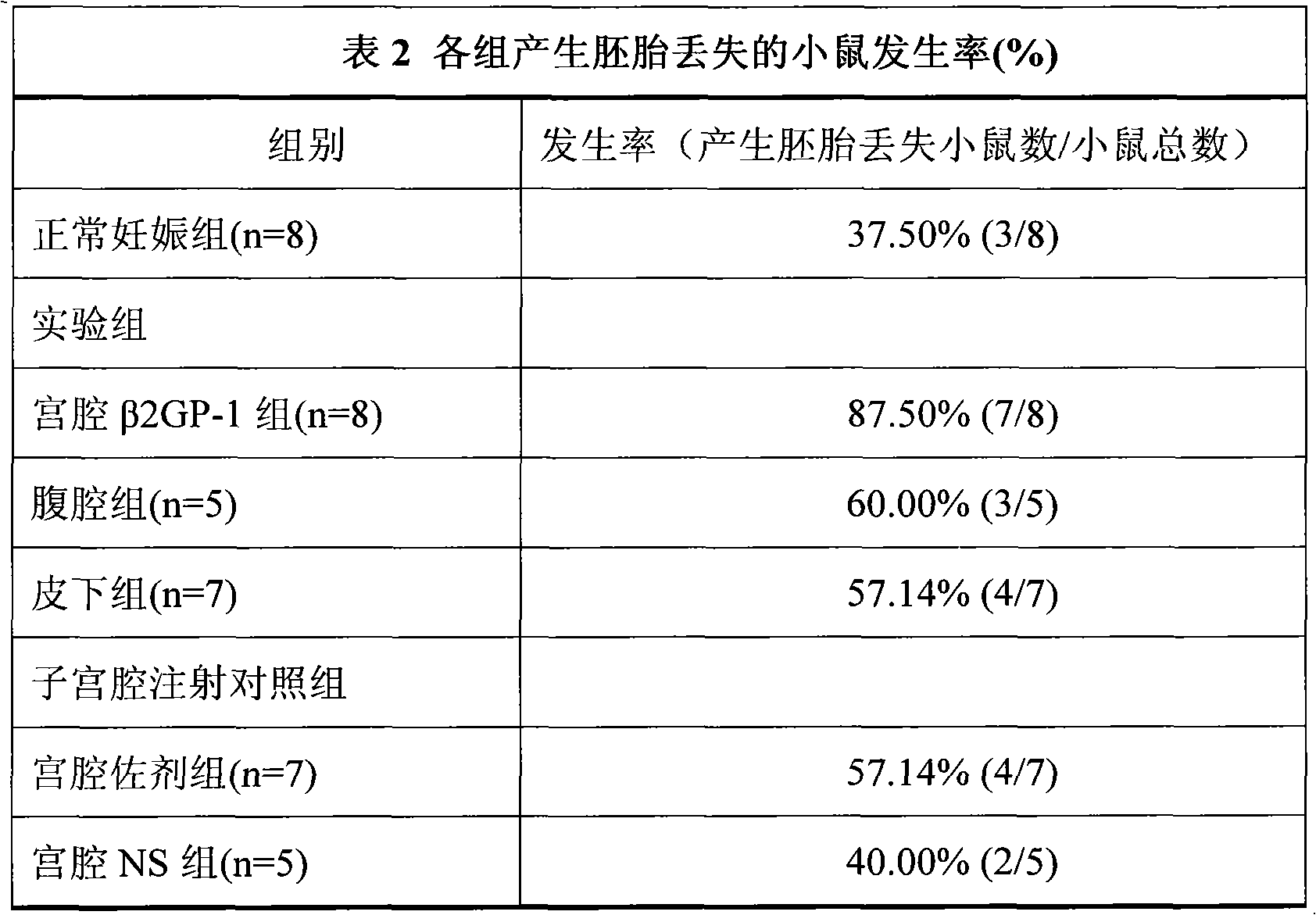
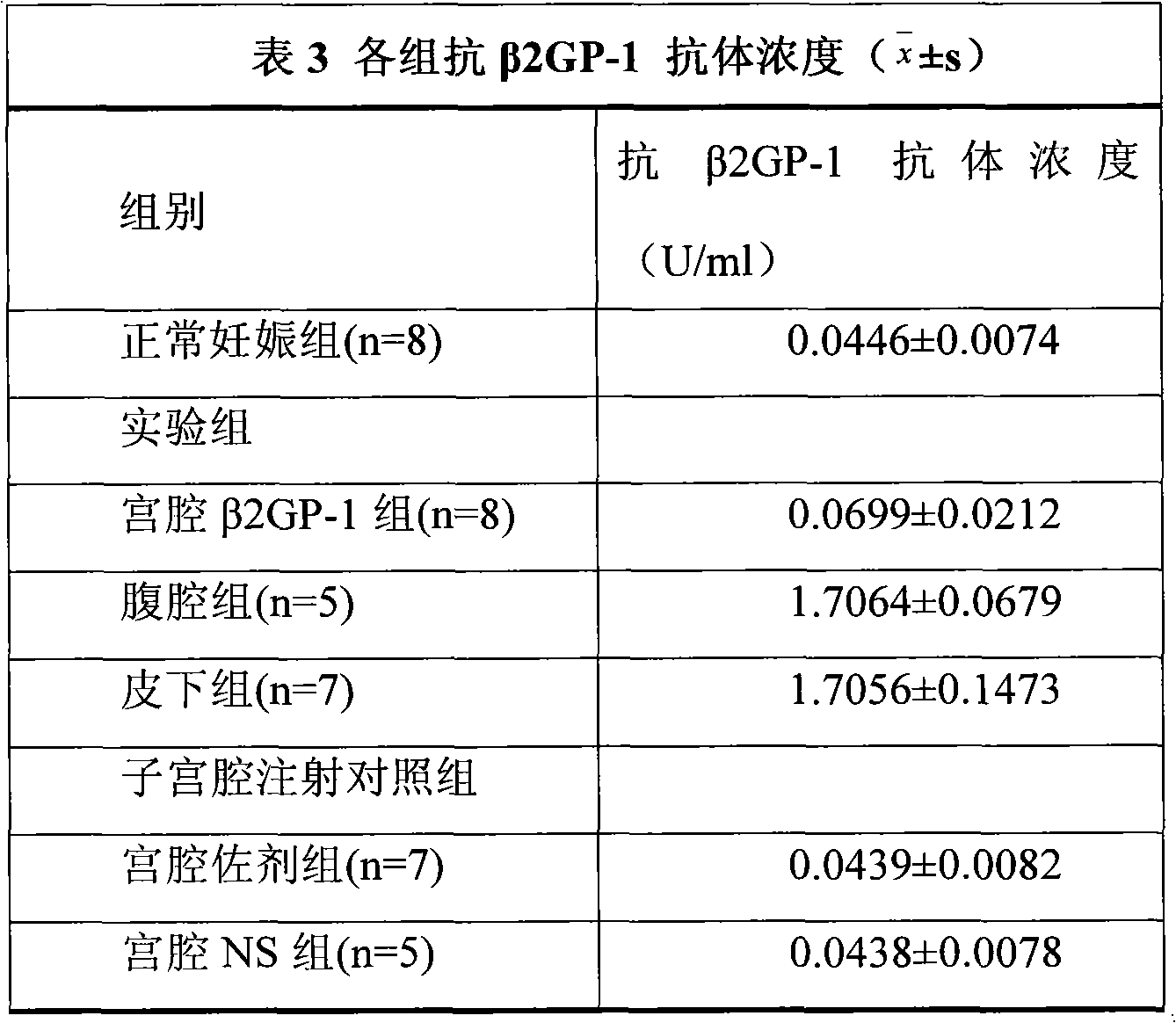
[0015] 40 female Balb / c mice and 20 males. Females were randomly divided into normal pregnancy group (n=8), intrauterine injection of β2GP-1 group (n=8), intraperitoneal injection of β2GP-1 group (n=5), subcutaneous injection of β2GP-1 group (n=7), uterine Intrauterine injection of adjuvant group (n=7) and intrauterine injection of NS group (n=5).
[0016] The normal pregnanc...
Embodiment 2
[0040] 1. Materials and methods
[0041] 1.1 Reagents
[0042] Human β2GP-1 (β2-glycoprotein-1) was purchased from Cell Sciences, USA; CFA (Freund's Adjuvant Complete) and IFA (Freund's Adjuvant Incomplete) were purchased from Sigma, USA; BALB / c mice were purchased from China Provided by the Shanghai Experimental Animal Center of the Academy of Sciences.
[0043] 1.2 Experimental animals
[0044]8-week-old clean-grade female and male BALB / c inbred mice, provided by the Shanghai Experimental Animal Center of the Chinese Academy of Sciences, weighing 20±2g.
[0045] 2. Construction of animal models
[0046] There were 37 female Balb / c mice and 19 males. Females were randomly divided into normal pregnancy group (n=7), intrauterine injection of β2GP-1 group (n=7), intraperitoneal injection of β2GP-1 group (n=5), subcutaneous injection of β2GP-1 group (n=7), uterine Intrauterine injection of adjuvant group (n=6) and intrauterine injection of NS group (n=5).
[0047] The norma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com