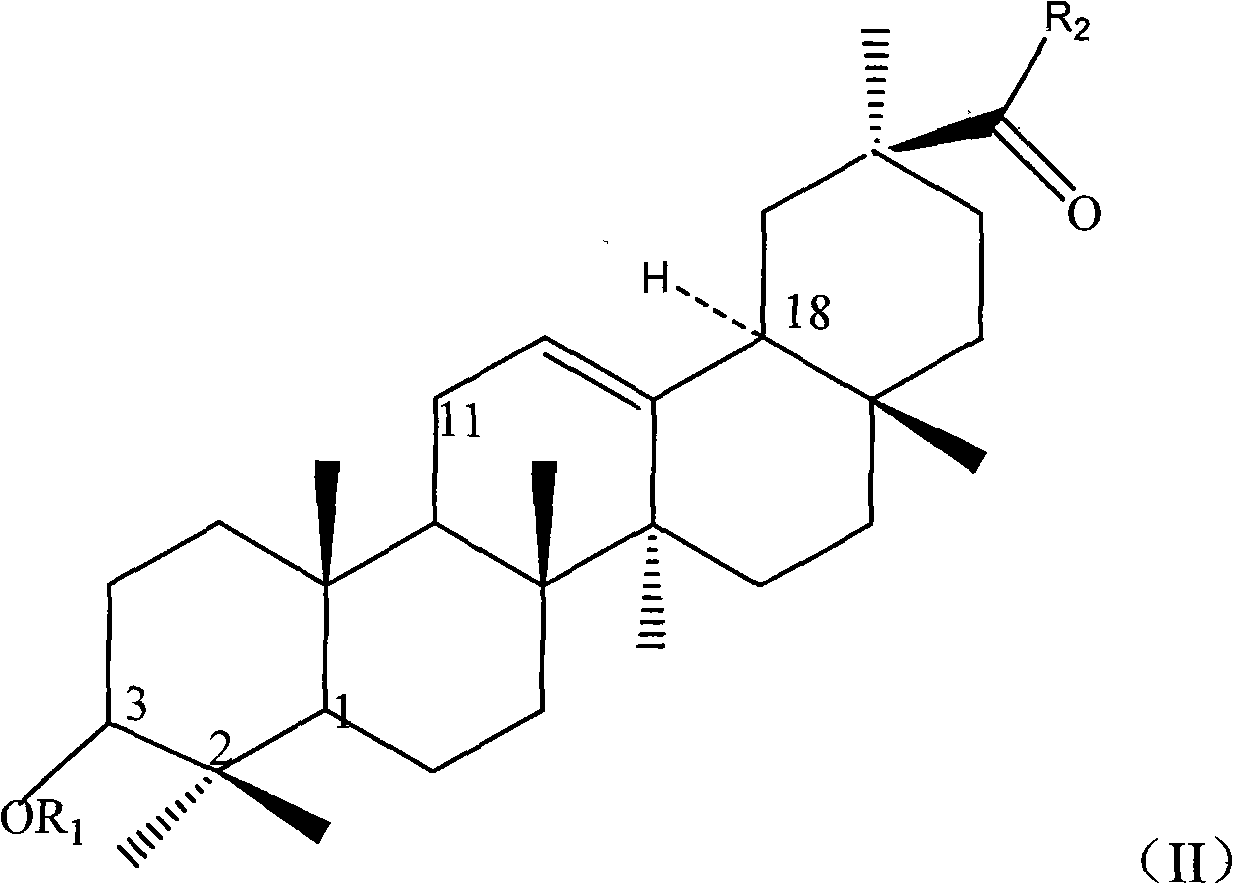
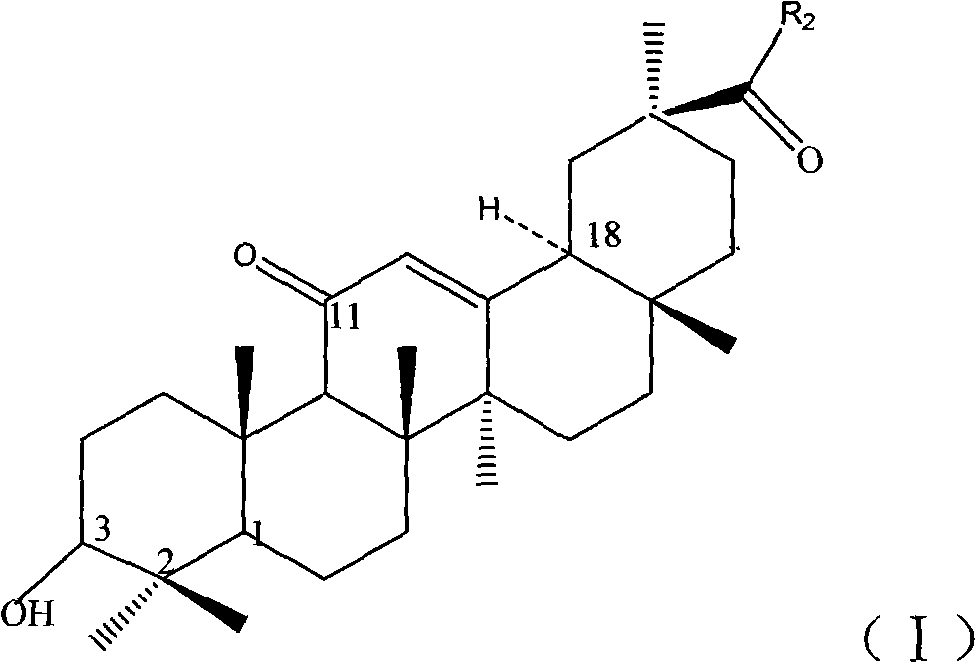
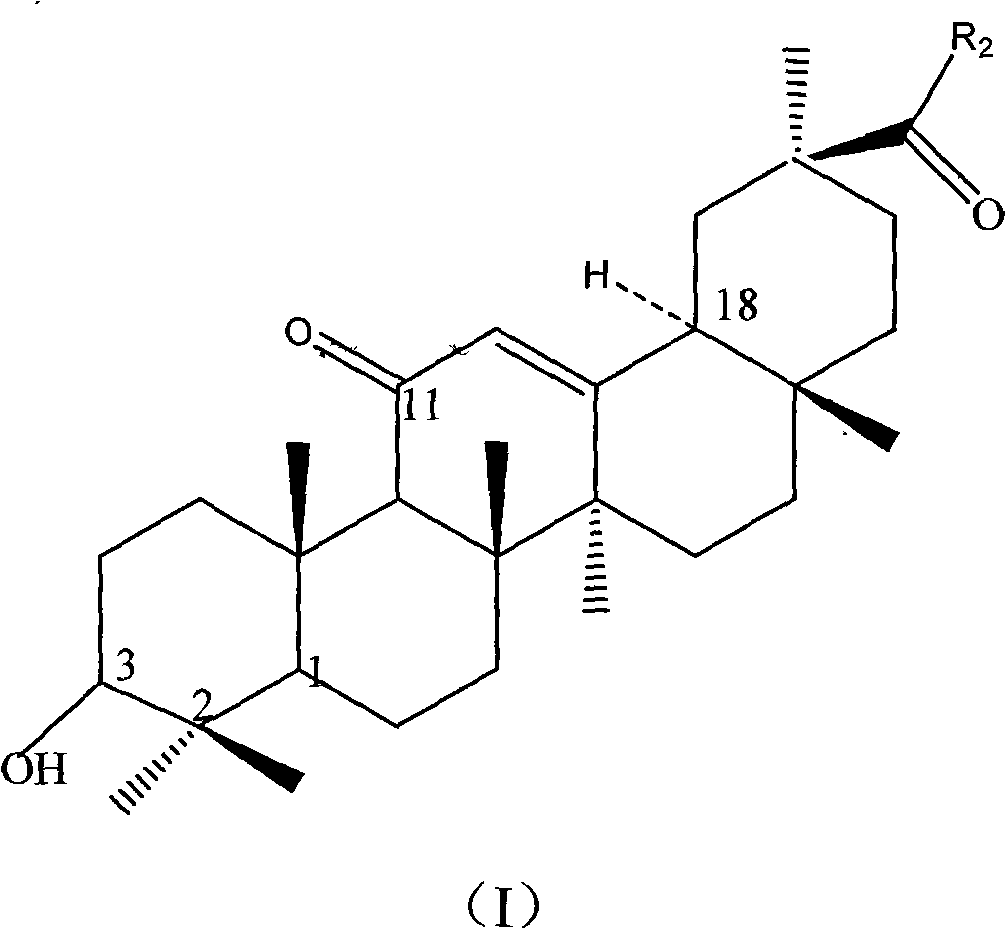
Synthetic method of glycyrrhetinic acid ester derivative and deoxyglycyrrhetinic acid ester compound
A technology of glycyrrhetinic acid and compounds, which is applied in the synthesis of glycyrrhetinic acid ester derivatives and the field of deoxyglycyrrhetinic acid ester compounds, can solve the problems of high equipment requirements, long reaction time, and unsuitability for industrial production, and achieve high drug efficacy , the effect of reducing waste of resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Synthesis of 18β-methyl glycyrrhetinate
[0043] Method 1. Add 10 g of 18β-glycyrrhizic acid to 100 ml of anhydrous methanol, add 5 ml of acetyl chloride, heat to reflux for 2 hours, add 100 ml of water, cool, crystallize a solid, filter, refine with ethanol / water, and dry to obtain the title compound.
[0044] Method 2. Add 20g of 18β-glycyrrhizic acid monoammonium salt into 100ml of anhydrous methanol, add 10ml of acetyl chloride, heat and reflux for 2 hours, the color turns brown, add 200ml of water, cool, crystallize a solid, filter, and refine with ethanol / water , dried to obtain the title compound in a yield of 79%.
[0045] IR: v as (-OH)3387cm -1 , v as (-COOCH 3 )1725cm -1 , v as (=O)1657, 1621cm -1 , v as (A district) 1387, 1361cm -1 , v as (Area B) 1322, 1278, 1246cm -1 .
Embodiment 2
[0046] Example 2 Synthesis of 18α-ethyl glycyrrhetinate
[0047] Method 1. Add 10 g of 18α-glycyrrhizic acid into 100 ml of absolute ethanol, add 5 ml of acetyl chloride, heat to reflux for 2 hours, add 100 ml of water, cool, crystallize a solid, filter, refine with 80% ethanol, and dry to obtain the title compound. Yield 85%.
[0048] 1 H-NMR: 0.72(s, 3H), 0.81(s, 3H), 1.00(s, 3H), 1.14(s, 3H), 1.20(s, 3H), 1.22(s, 3H), 1.26(t, 3H), 1.35(s, 3H), 4.14(q, 2H), 5.57(s, 1H)
[0049] 13 C-NMR (ppm): 14.13, 15.62, 15.94, 16.47, 17.54, 18.49, 20.65, 20.75, 26.65, 27.22, 28.07, 28.40, 31.70, 33.75, 35.45, 35.97, 36.84, 37.60, 39.0792, 40.39. , 43.80, 44.89, 54.99, 60.42, 60.66, 78.70, 124.08, 165.64, 178.20, 199.74
[0050] Method 2. Add 10g of 18α-glycyrrhizic acid into 100ml of absolute ethanol, add 1ml of concentrated sulfuric acid, heat and reflux for 8 hours, add 100ml of water, cool, crystallize a solid, filter, refine with ethanol / water, and dry to obtain the title compou...
Embodiment 4
[0051] Example 4 Synthesis of ethyl 11-deoxy-18α glycyrrhetinate
[0052] Add 11 g of ethyl 18α-glycyrrhetinate and 6 g of zinc powder into 150 ml of 1,4-dioxane, add a little water, pass in hydrogen chloride gas, and stir for 5 hours. Filter, evaporate the solvent from the mother liquor, add 50ml of water and 100ml of ethyl acetate, stir, separate layers, wash the organic layer with water, evaporate to dryness, and refine with ethanol / water to obtain 8.6g of white crystals.
[0053] IR: v as (-OH)3374cm -1 , v as (-COOCH 3 )1727cm -1 , v as (A district) 1382cm -1 , v as (Area B) 1300, 1278cm -1 .
[0054] 1 H-NMR: 0.66(s, 3H), 0.79(s, 3H), 0.96(s, 3H), 0.99(s, 3H), 1.00(s, 3H), 1.15(s, 3H), 1.22(s, 3H), 1.25(t, 3H), 4.12(q, 2H), 5.18(t, 1H)
[0055] 13 C-NMR (ppm): 14.19, 15.24, 15.69, 15.83, 17.44, 18.30, 20.93, 23.17, 23.17, 26.28, 27.27, 28.14, 28.73, 32.38, 34.15, 34.96, 36.07, 36.86, 38.661, 38.8 , 39.55, 42.70, 43.67, 47.24, 55.31, 60.20, 79.02, 117.55, 14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com