Construction of a long-acting polypeptide and its application in anti-acute kidney injury and diabetic nephropathy
A technology for acute kidney injury and diabetes, which is applied in the field of biotechnology drugs, can solve the problems of weak binding ability between peptides and receptors, short half-life, lack of effective treatment methods for diabetic nephropathy, etc., and achieve the effect of prolonging plasma half-life and enhancing binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Solid-phase synthesis and purification methods for polypeptides used in the present invention.
[0054] Swell 1 g of Fmoc-rink AM resin (sample loading: 0.3 mmol / g) in 10 mL of DMF for 4 hours, remove the DMF, add DMF containing 20% piperidine, react with shaking at room temperature for 45 min, and remove the Fmoc protecting group. The deprotected resin was washed with DMF to remove residual piperidine. Then add 15 mL of DMF solution containing Fmoc amino acid (3.0 equivalents), HBTU (2.9 equivalents), HOBt (3.0 equivalents), DIPEA (6.0 equivalents), shake at room temperature for 2 hours, remove the reaction solution, clean the resin with DMF, and perform Kaiser test Whether the reaction is complete (if the reaction is not complete, repeat the coupling once). According to the amino acid sequence of the polypeptide, the coupling of amino acids is carried out from the C-terminal to the N-terminal. After the polypeptide sequence is completed, use DMF, CH 2 Cl 2 , MeOH...
Embodiment 2
[0058] The in vitro plasma half-life of the K(pal)-E11 peptide, PEG-E11 peptide, Pal-E11 peptide and Dimer-E11 peptide of the present invention is longer than that of the E11 peptide.
[0059] Specific operation: 600 μL of fresh plasma, 60 μg of polypeptide, add 3000 μL of Tris-HCl buffer, incubate at 37 degrees Celsius, take 600 μ of reaction solution each time at the six time points of 0, 2, 4, 8, 12 and 24 hours, and transfer to the taken out Add 30 μL TFA to the reaction solution sample to terminate the reaction, then add 3000 to 4000 μL methanol and mix thoroughly - let it stand for 20 minutes, centrifuge at 10000 rpm for 5 minutes, take the supernatant, process it in a freeze concentrator, evaporate the liquid, and add methanol / water containing 0.1% TFA (1:1) 20 microliters, fully dissolved and centrifuged at 10,000 rpm for 5 minutes, the supernatant was taken for HPLC analysis, and the peak area was integrated to determine the half-life. The experimental results are sho...
Embodiment 3
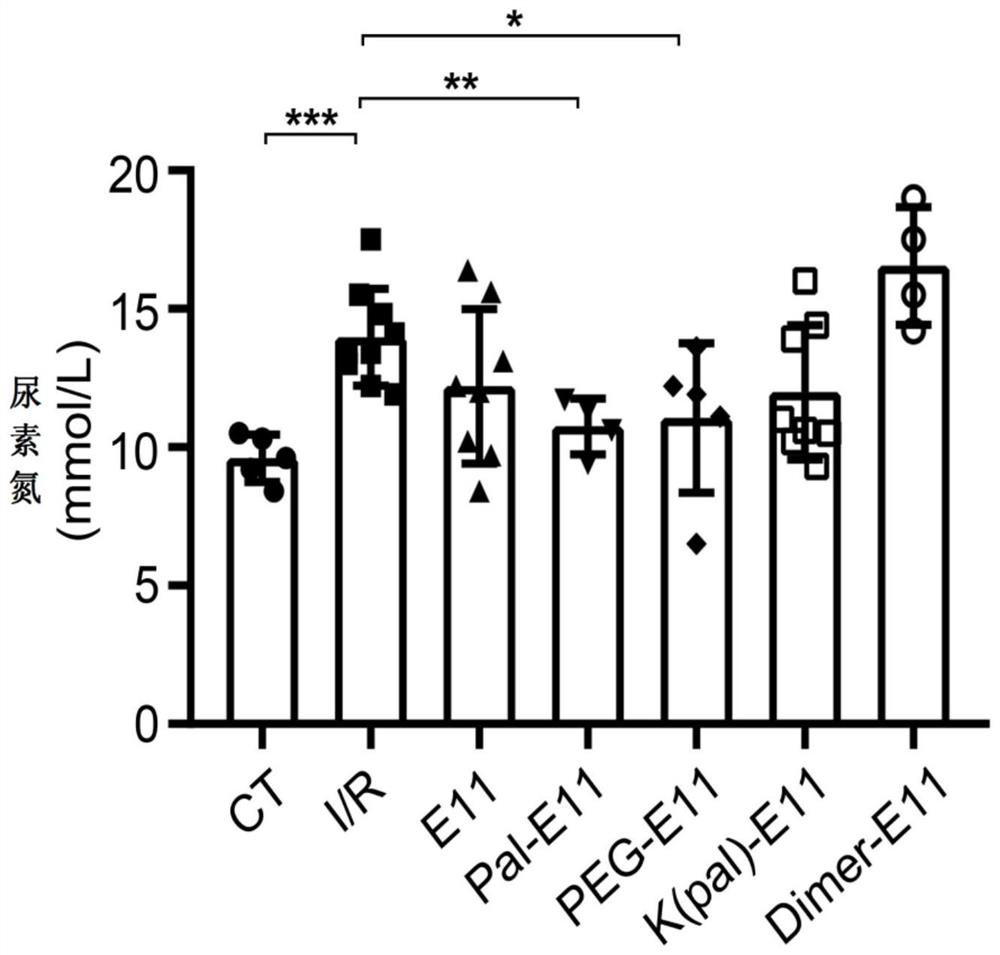
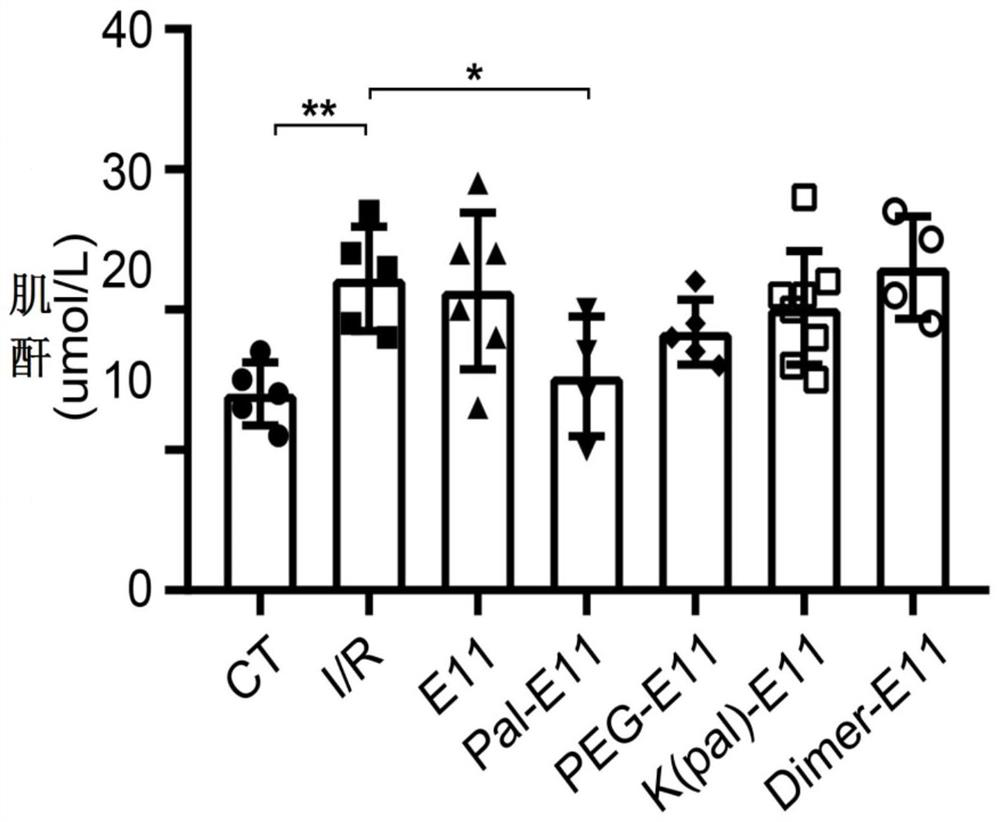
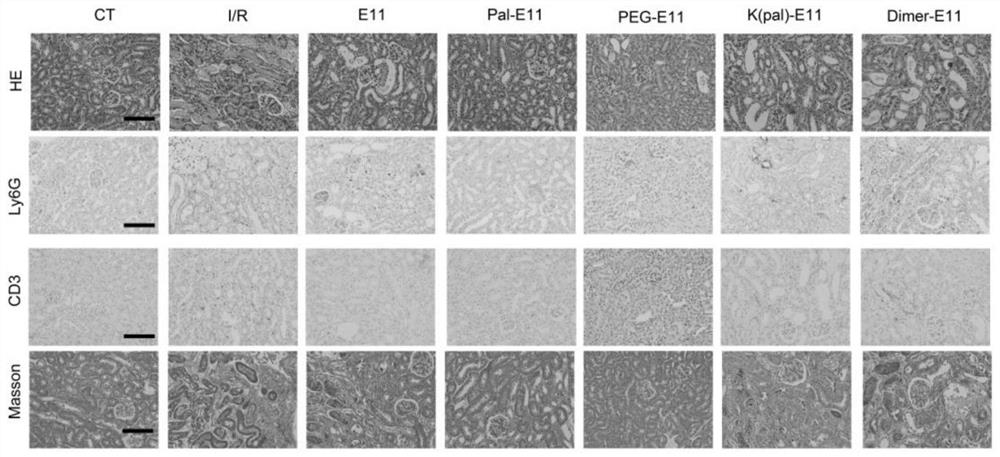
[0063] The PEG-E11 peptide and Pal-E11 peptide of the present invention can inhibit renal dysfunction caused by renal I / R injury.
[0064] Specific operation: Before the formal experiment, we conducted a series of polypeptide injection dose pre-experiments of the present invention. After the injection of the series of polypeptides, the physiological indicators of the mice were normal in all aspects, and the series of polypeptides were considered to be safe. Mice weighing about 25g were divided into: control mouse group (CT group), renal ischemia-reperfusion mouse group (I / R group), and renal ischemia-reperfusion mouse group given E11 peptide (E11 group) , renal ischemia-reperfusion mouse group was given K(pal)-E11 peptide group (K(pal)-E11 group) peptide, renal ischemia-reperfusion mouse group was given Pal-E11 peptide group (Pal-E11 group), The renal ischemia-reperfusion mouse group was given PEG-E11 peptide group (PEG-E11 group) and the renal ischemia-reperfusion mouse group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com