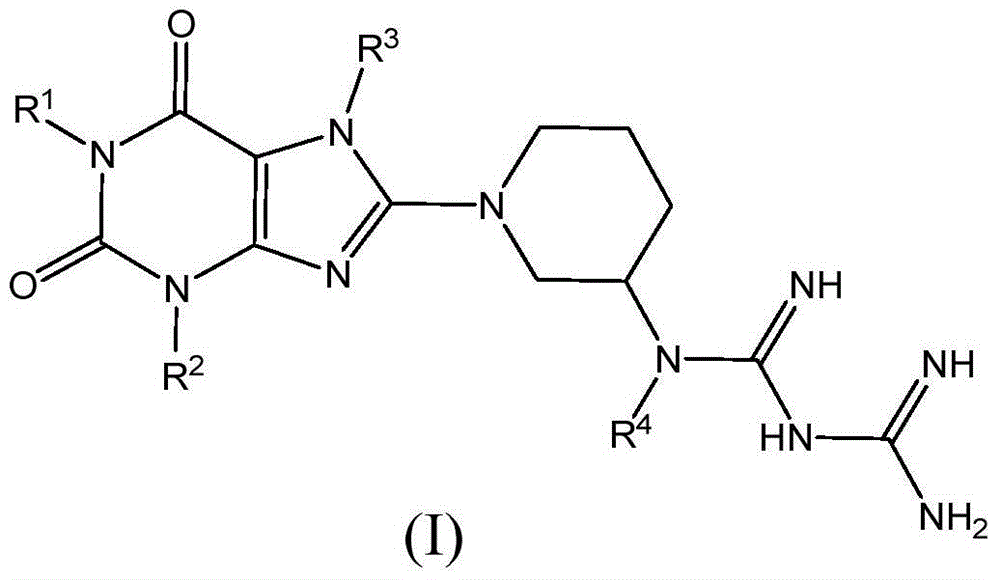
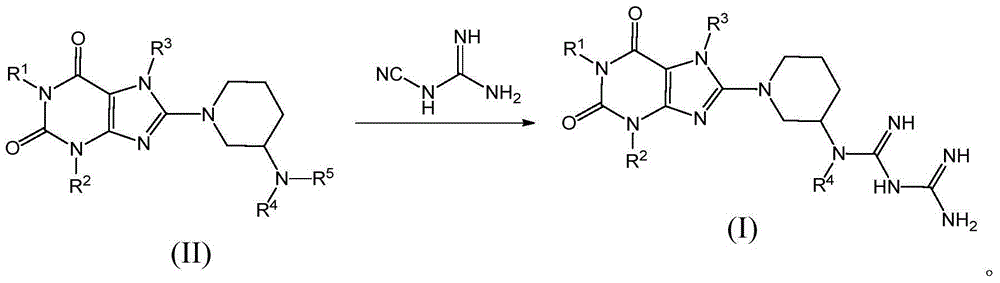
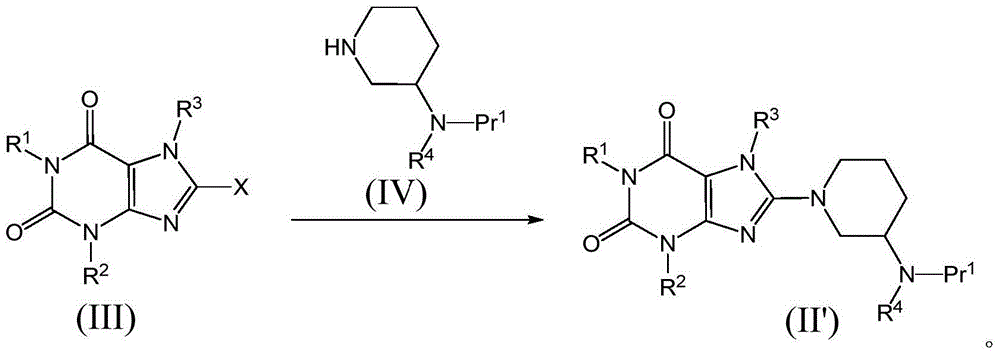
Compound as dipeptidyl peptidase-4 inhibitor
A compound and alkyl technology, applied in the field of compounds as dipeptidyl peptidase-4 inhibitors, can solve the problems of lack of hypoglycemia, body weight, etc., and achieve the effect of improving or restoring function and preventing β-cell degeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The preparation of formula (III) compound
[0061] 1.8-Bromo-7-(2-butyn-1-yl)-3-methyl-1-((4-methylquinazolin-2-yl)methyl)-3,7-xanthine (compound III-1) Preparation
[0062]
[0063] (1) Preparation of 3-methyl-7-(2-butyn-1-yl)-8-bromo-3,7-xanthine
[0064] Mix 3-methyl-8-bromo-xanthine with N-ethyldiisopropylamine (DIPEA) in N,N-dimethylformamide, add 1-bromo-2-butyne, and Stir overnight at room temperature. For work-up, the reaction mixture was poured into water. The separated precipitate is suction filtered, washed with water, and dried to obtain 3-methyl-7-(2-butyn-1-yl)-8-bromo-xanthine (molecular formula: C 10 h 9 BrN 4 o 2 ).
[0065] Mass spectrometry (ESI + ): m / z=298[M+H] +
[0066] Elemental analysis: C, 40.43; H, 3.05; Br, 26.89; N, 18.86; O, 10.77
[0067] (2) Preparation of 8-bromo-7-(2-butyn-1-yl)-3-methyl-1-((4-methylquinazolin-2-yl)methyl)-3,7-xanthine
[0068] Add 2-(2-bromoethyl)-4-methylquinazoline to the N of 3-methyl-7-(2-buty...
Embodiment 1
[0169] Example 1: Preparation of l-((4-methyl-quinazolin-2-yl)methyl)-3-methyl-7-(2-butyn-1-yl)-8-((R) -3-(N1-n-Butylbiguanide)-piperidin-1-yl)-xanthine (Compound I-1)
[0170]
[0171] Dissolve dicyandiamide in isopropanol, add (R)-8-(3-n-butylaminopiperidin-1-yl)-7-(2-butyn-1-yl)-3-methyl -1-((4-methylquinazolin-2-yl)methyl)-3,7-xanthine (compound II-1), adjust the pH to 5-6 with 36% HCl, and control the temperature at 80-100 ℃ for 6 hours. After the reaction was completed, it was allowed to stand for cooling, and a large amount of white crystals were precipitated. After suction filtration, washing, drying, and recrystallization with absolute ethanol, the title compound (molecular formula: C 31 h 40 N 12 o 2 ;).
[0172] Mass spectrometry (ESI + ): m / z=613[M+H] + ;
[0173]Elemental analysis: C, 60.77; H, 6.58; N, 27.43; O, 5.22;
[0174] 1 H-NMR (d6-DMSO): 8.12(1H), 7.85(2H, br), 7.80(2H), 7.59(1H), 6.63(2H, br), 4.43(4H), 3.59(1H), 3.38( 3H), 3.34(1H), 3.30(2...
Embodiment 2
[0175] Example 2: l-(dimethylaminocarbonylmethyl)-3-methyl-7-(1-cyclohexenylmethyl)-8-((R)-3-(N1-cyclohexylbiguanide) Preparation of -piperidin-1-yl)-xanthine (compound 1-2)
[0176]
[0177] Dissolve dicyandiamide in isopropanol, add (R)-8-(3-cyclohexylaminopiperidin-1-yl)-7-(1-cyclohexenylmethyl)-3-methyl- 1-((4-methylquinazolin-2-yl)methyl)-3,7-xanthine (compound II-2), adjust the pH to 5-6 with 36% HCl, and control the temperature at 80-100°C Reacted for 7 hours, stood still after the completion of the reaction, cooled and crystallized, filtered with suction, washed, dried and recrystallized with absolute ethanol to obtain the title compound (molecular formula: C 30 h 47 N 11 o 3 ).
[0178] Mass spectrometry (ESI + ): m / z=610[M+H] + ;
[0179] Elemental analysis: C, 59.09; H, 7.77; N, 25.27; O, 7.87.
[0180] 1 H-NMR (d6-DMSO): 7.84 (2H, br), 6.63 (2H, br), 5.39 (1H), 4.40 (2H), 4.28 (2H), 3.59 (1H), 3.42 (3H), 3.34 ( 1H), 3.27(2H), 2.98(6H), 2.64(1H), 2.56(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com