Application of citral in preparation of medicament for treating biliary tract diseases
A technology of citral and medicine, which is applied in the application field of citral in the preparation of medicines for treating biliary tract diseases, and can solve the problems of undeveloped medicines and no reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0020] Experimental Example 1: Promoting bile secretion in rats and its effects on bile components
[0021] 1. The effect of one administration
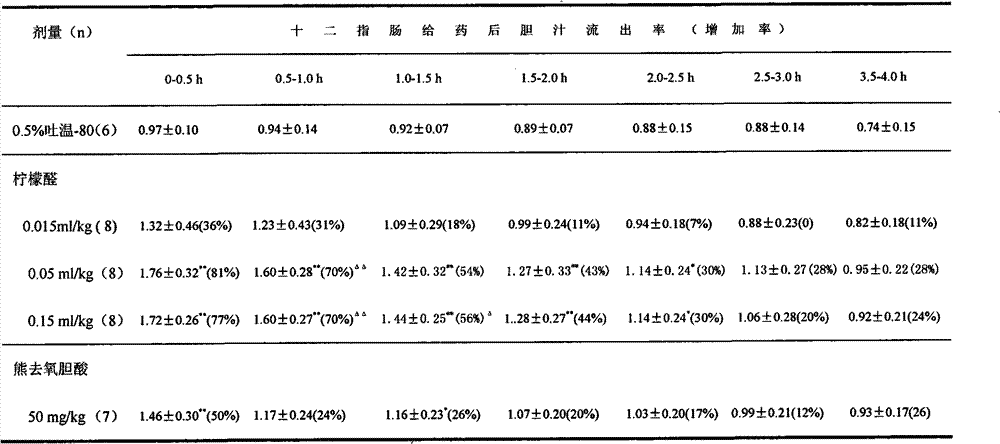
[0022] Thirty-seven male rats, weighing 219±15g, were divided into 5 groups. After urethane anesthesia, bile duct intubation was performed, and after 0.5 hours of equilibration, the bile flow before the drug was collected for 0.5 hours, and collected after duodenal administration of citral, ursodeoxycholic acid or 0.5% Tween-80 Every 0.5h of flow, continuous 4h. Comparing the flow of every 0.5h with the flow before drug (flow after drug / flow before drug), the outflow rate can be obtained. Then compared with the corresponding phase of the control group by t test, the results are shown in Table 1.
[0023] Table 1 The effect of one-time duodenal administration of citral on bile secretion in anesthetized rats (M ± S)
[0024]
[0025] * p** p△ p△△ p<0.01 (compared with ursodeoxycholic acid group).
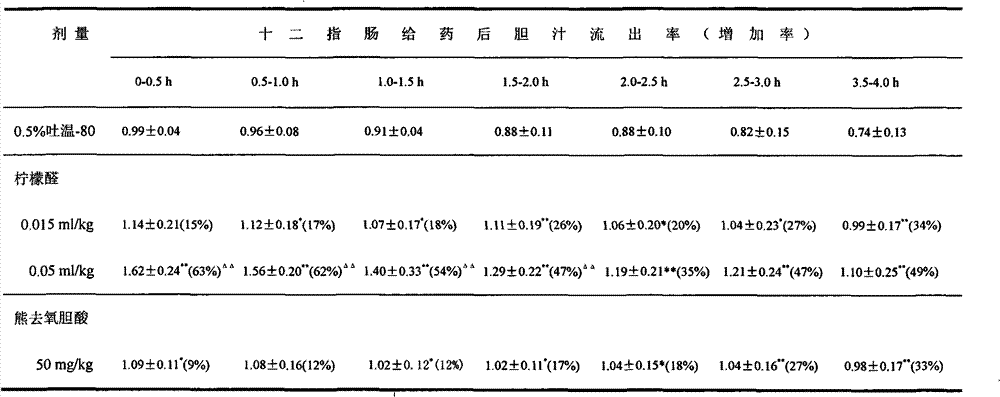
[0026] 2. The effect of repe...
experiment example 2
[0036] Experimental Example 2: Inhibition of Gallstone Formation in Mice
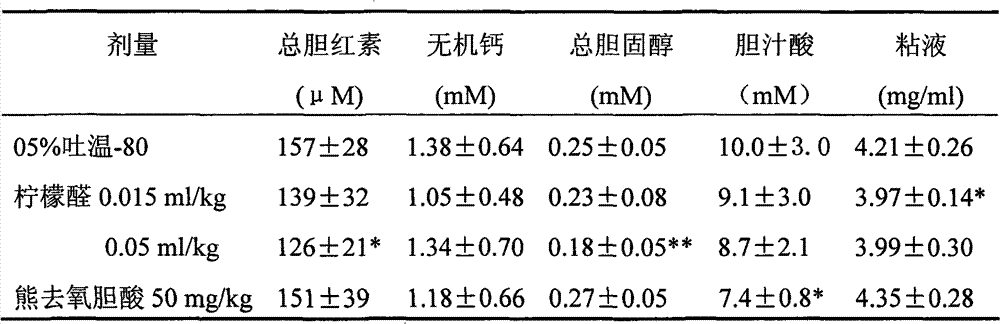
[0037]104 mice, half male and half male, weighing 18.8±1.6g, were divided into 5 groups. Except for the blank control group fed with normal diet, the mice in the other 4 groups were fed with stone-causing diet. Each animal was fed with 6 g of feed per day. The formulation of the stone-causing feed is: add 1% cholesterol and 0.5% cholic acid to the normal feed. The medication group was fed with citral or ursodeoxycholic acid, while the model-making control group was fed with solvent 0.5% Tween-80. During the 4th week of the experiment, blood was collected and the gallbladder was removed under anesthesia, the serum total cholesterol content and serum alanine aminotransferase level were measured, and gallstone formation in the gallbladder was observed. From the 4-week experimental results in Table 4, it can be seen that both ursodeoxycholic acid and citral have the effect of inhibiting the formation of ...
experiment example 3
[0041] Experimental Example 3: Dissolving human gallstones in a test tube
[0042] The tested drugs were all dissolved with Tween-80, and the content of Tween-80 in the prepared suspension was 0.5%. All medicinal solutions were adjusted to pH 8.5 with 0.4% NaOH. The human mixed gallstones obtained from surgery were washed and placed in an oven at 60°C with constant weight, and then arranged according to shape, color and weight, and then randomly divided into groups. Put a gallstone into a stoppered test tube, add 1ml of the test drug, shake it 3-4 times a day, change the drug solution once a day, take out the gallstone after 2 weeks, wash it, put it in an oven at 60°C, and dry it Weigh after the constant weight, and the difference between the gallstones weighed twice is the weight of the gallstones dissolved. Data were subjected to between-group t-tests (Table 5). The results showed that both the citral 0.5ml% group and the ursodeoxycholic acid 0.5g% group could dissolve hu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com