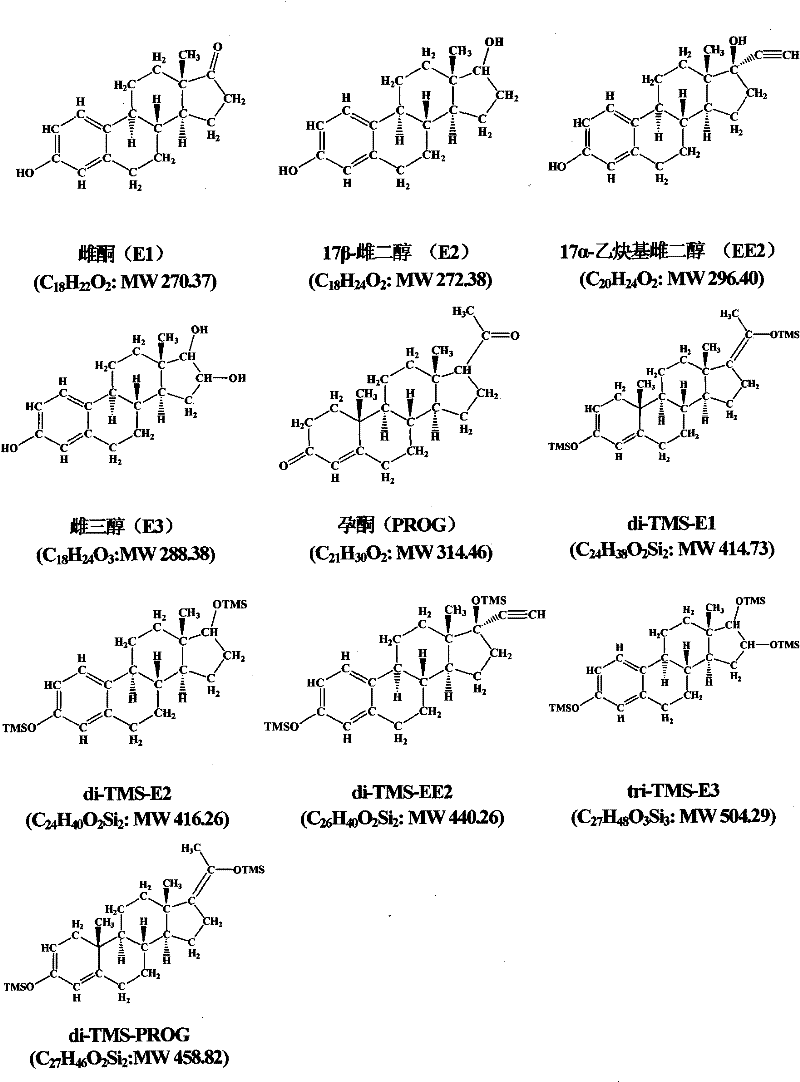
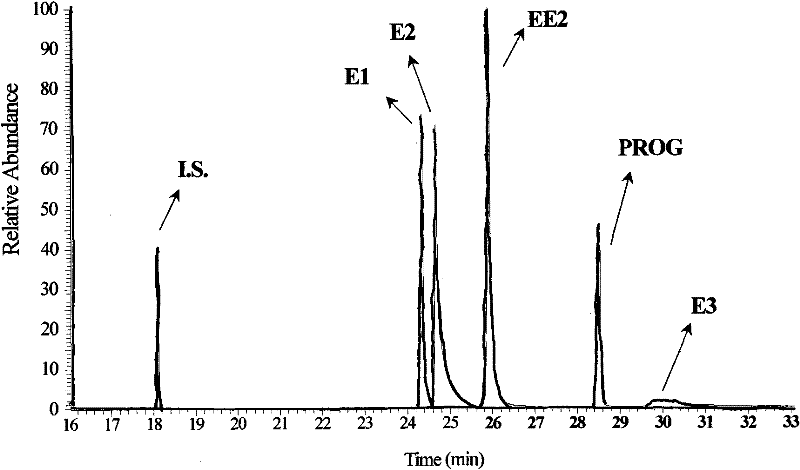
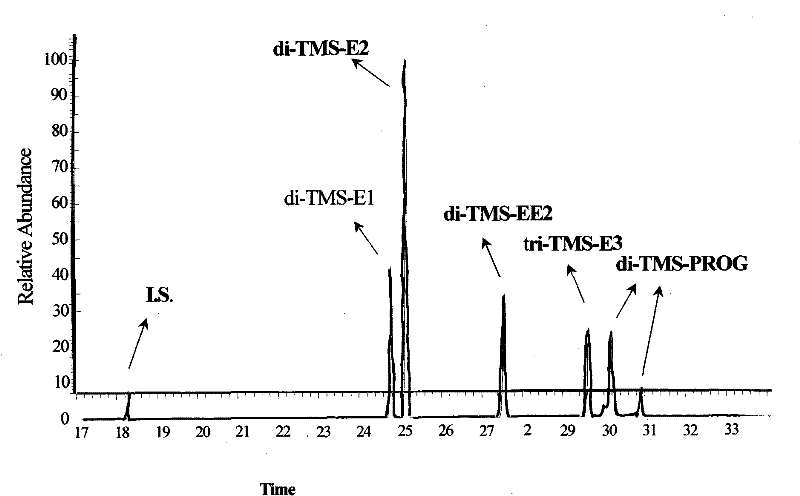
Hydroxyl group/keto group synchronous derivatization method of endocrine disturbing chemicals in steroid environment
A technology based on endocrine disruptors and hydroxyketones, which is applied in the field of environmental analytical chemistry, can solve the problems of large difference in the amount of catalyst added, increased energy consumption and cost, and reduced detection accuracy, so as to save heating steps and reduce energy consumption and cost, the effect of reducing experimental cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The concrete steps of hydroxyketone synchronous derivatization method are:
[0036] 1. First, accurately weigh 10 mg of E1, E2, EE2, E3, PROG, and internal standard into 10 mL chromatographic bottles, respectively add 10 mL of methanol, shake for 60 s, and prepare standard stock solutions with a concentration of 1 μg / μL, seal Store at -20°C for later use; use methanol and standard stock solution to prepare a standard mixed solution containing E1, E2, EE2, E3, PROG at a concentration of 5pg / L and an internal standard at a concentration of 100pg / μL;
[0037] 2. Take 100 μL of the standard mixed solution prepared in step (1) in a 1.5 mL chromatographic bottle, put the chromatographic bottle into a nitrogen blower, and blow dry slowly with nitrogen gas with a purity of 99.99%;
[0038] 3. Add 100 μL of MSTFA / TMIS / DTE (1000:5:5, v / v / w), shake for 30 seconds to mix, and place in a dry and heated environment at 20°C for 10 minutes to react;
[0039] 4. Take out and cool to ro...
Embodiment 2
[0042] The concrete steps of hydroxyketone synchronous derivatization method are:
[0043] 1. First, accurately weigh 10 mg of E1, E2, EE2, E3, PROG, and internal standard into 10 mL chromatographic bottles, respectively add 10 mL of methanol, shake for 60 s, and prepare standard stock solutions with a concentration of 1 μg / μL, seal Store at -30°C for later use; use methanol and standard stock solution to prepare a standard mixed solution containing E1, E2, EE2, E3, PROG at a concentration of 5pg / L and an internal standard at a concentration of 100pg / μL;
[0044] 2. Take 100 μL of the standard mixed solution prepared in step (1) in a 1.5 mL chromatographic bottle, put the chromatographic bottle into a nitrogen blower, and blow dry slowly with nitrogen gas with a purity of 99.99%;
[0045] 3. Add 100 μL of MSTFA / TMIS / DTE (1000:5:5, v / v / w), shake for 30 seconds to mix, and place in a dry and heated environment at 20°C for 5 minutes to react;
[0046] 4. Take out and cool to roo...
Embodiment 3
[0049] The concrete steps of hydroxyketone synchronous derivatization method are:
[0050] 1. First, accurately weigh 10 mg of E1, E2, EE2, E3, PROG, and internal standard into 10 mL chromatographic bottles, respectively add 10 mL of methanol, shake for 60 s, and prepare standard stock solutions with a concentration of 1 μg / μL, seal Store at -40°C for later use; use methanol and standard stock solution to prepare a standard mixed solution containing E1, E2, EE2, E3, PROG at a concentration of 5pg / L and an internal standard at a concentration of 100pg / μL;
[0051] 2. Take 100 μL of the standard mixed solution prepared in step (1) in a 1.5 mL chromatographic bottle, put the chromatographic bottle into a nitrogen blower, and blow dry slowly with nitrogen gas with a purity of 99.99%;
[0052] 3. Add 100 μL of MSTFA / TMIS / DTE (1000:5:5, v / v / w), shake for 30 seconds to mix, then place in a dry and heated environment at 30°C for 7 minutes to react;
[0053] 4. Take out and cool to ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com