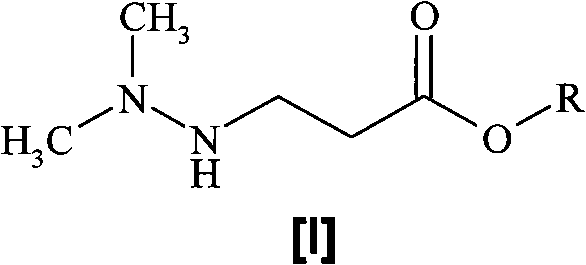
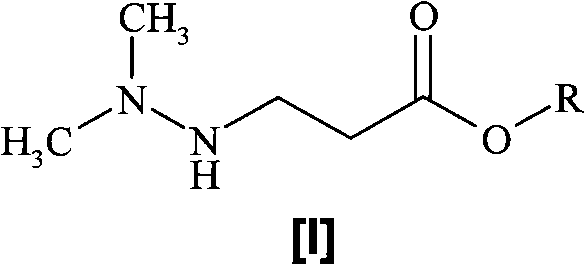
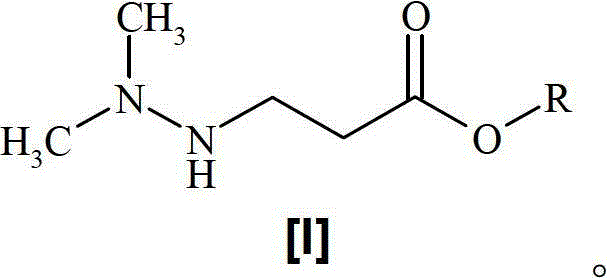
A one-pot process for preparing 3-(2,2,2-trimethylhydrazinium)propionate dihydrate
A technology of dihydrate and trimethylhydrazine, applied in the preparation of hydrazine, organic chemistry, etc., can solve the problems of the standard alkali hydrolysis of carbonates and sulfates, etc., and achieve the effect of increasing yield and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of 3-(2,2,2-trimethylhydrazine)propionate dihydrate
[0019] Dissolve methyl 3-(2,2-dimethylhydrazino)propionate (146 g, 1 mol) in water (290 mL) and heat at 90-100 °C under an inert atmosphere until the starting ester Complete conversion to the corresponding acid (monitored by HPLC). Methanol (150 mL) was added to the reaction mixture, followed by dimethyl carbonate (843 mL, 10 mol). The reaction mixture was heated in the reactor at 90 °C until completion of the reaction (monitored by HPLC). Methanol, dimethyl carbonate and water were removed by distillation under reduced pressure. The distillation residue was dissolved in hot isopropanol (500ml) and evaporated again. The obtained 3-(2,2,2-trimethylhydrazine)propionate dihydrate was dried under reduced pressure. Yield 172 g (94.4%).
Embodiment 2
[0021] Preparation of 3-(2,2,2-trimethylhydrazine)propionate dihydrate
[0022] Dissolve methyl 3-(2,2-dimethylhydrazino)propionate (146 g, 1 mol) in water (270 mL), then add methanol (170 mL) and dimethyl carbonate (843 mL, 10 mol) to the solution ). The reaction mixture was heated in the reactor at 95°C until completion of the reaction (monitored by HPLC). Methanol, dimethyl carbonate and water were removed by distillation under reduced pressure. The distillation residue was dissolved in hot isopropanol (500ml) and evaporated again. The obtained 3-(2,2,2-trimethylhydrazine)propionate dihydrate was dried under reduced pressure. Yield 171 g (93.9%).
Embodiment 3
[0024] Preparation of 3-(2,2,2-trimethylhydrazine)propionate dihydrate
[0025] 3-(2,2-Dimethylhydrazino)propionic acid (132 g, 1 mol) was dissolved in water (180 mL), then methanol (170 mL) and dimethyl carbonate (843 mL, 10 mol) were added to the reaction mixture ). The reaction mixture was heated in the reactor at 95°C until completion of the reaction (monitored by HPLC). Methanol, dimethyl carbonate and water were removed by distillation under reduced pressure. The distillation residue was dissolved in hot isopropanol (500ml) and evaporated again. The obtained 3-(2,2,2-trimethylhydrazine)propionate dihydrate was dried under reduced pressure. Yield 175 g (96.0%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com