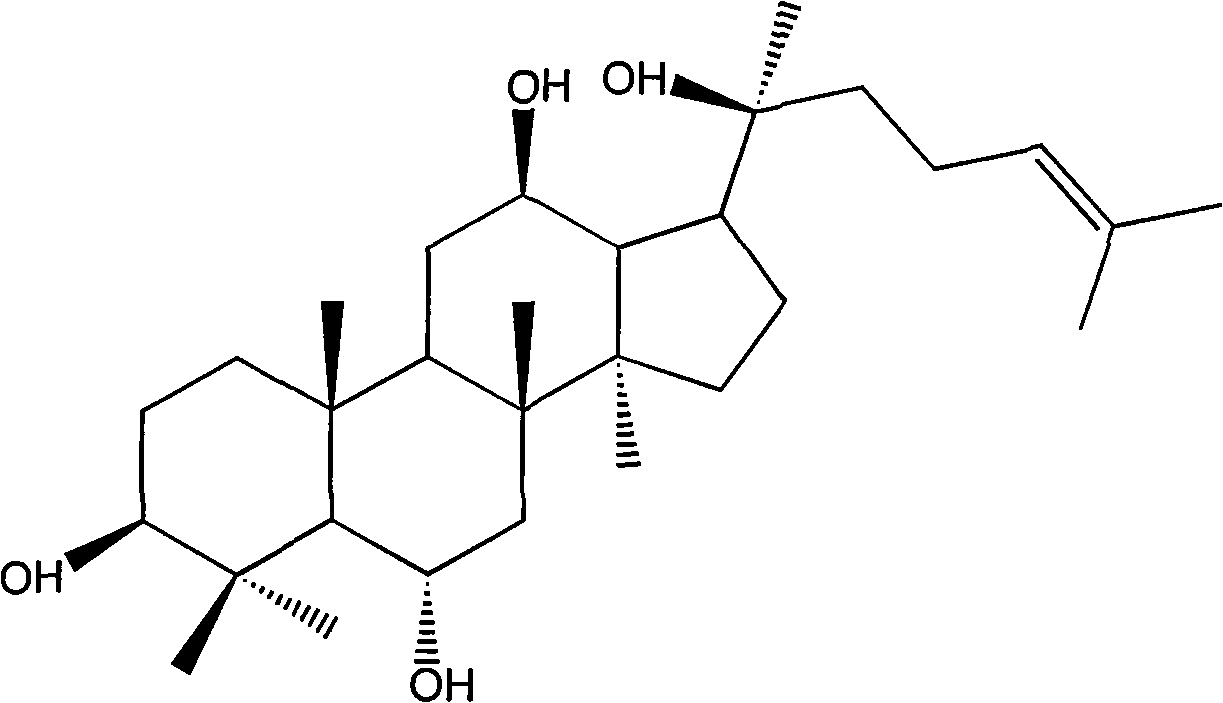
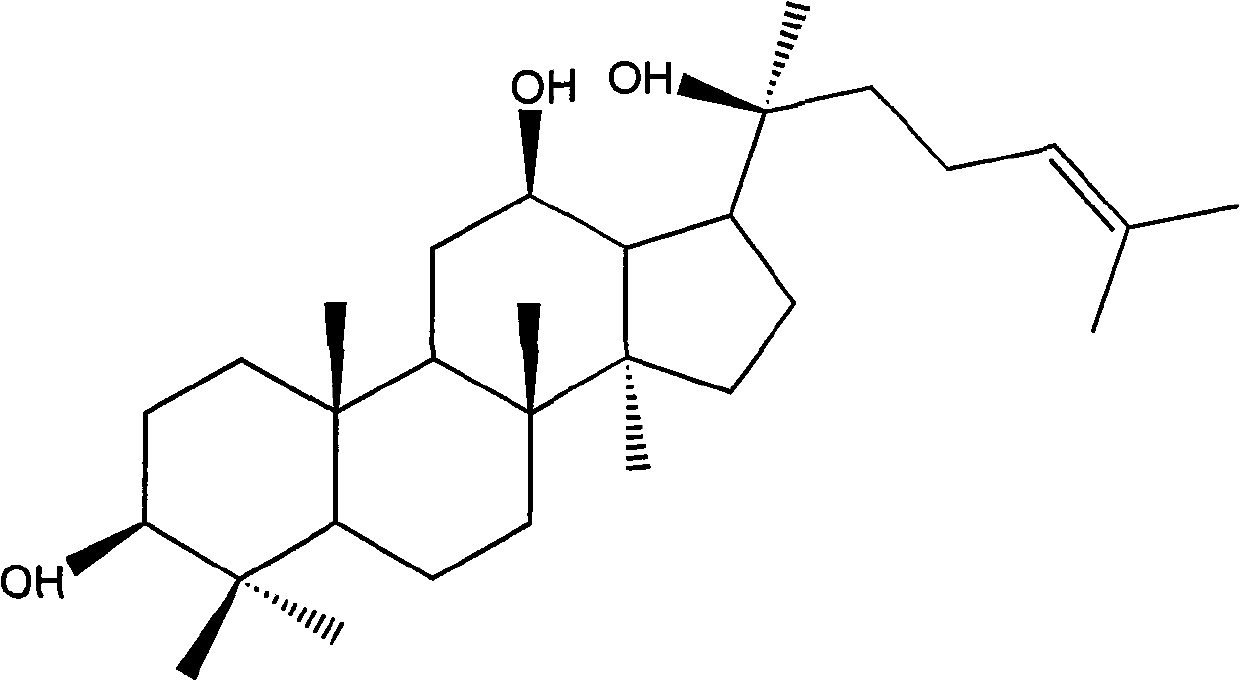
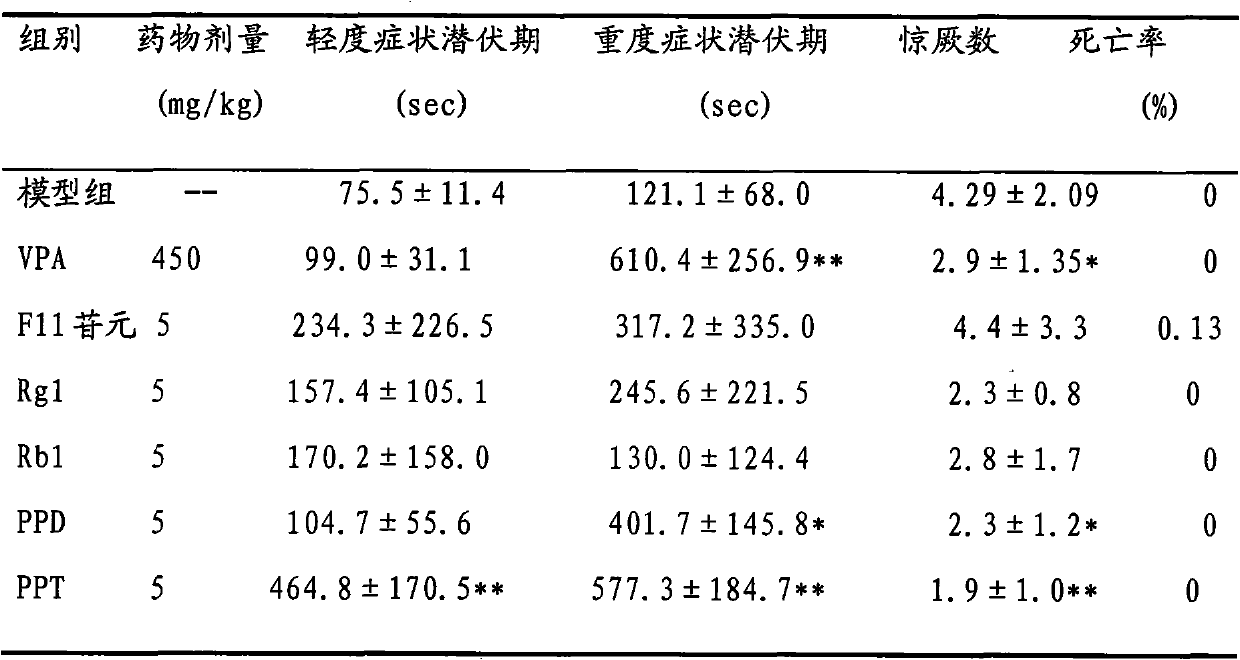
Medical application of protopanaxatriol and protopanaxadiol in nervous system diseases
A technology for nervous system diseases and protopanaxadiol, which is applied in the field of medical applications, can solve the problems of less research on ginsenosides and less reports on the pharmacological activity of nervous system diseases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment one: the preparation of PPD, PPT, ginsenoside mixture tablet and compound tablet
[0070] 1. PPT tablet prescription (1000 tablets):
[0071]
[0072] Prepared using common techniques.
[0073] 2. PPT tablet prescription (1000 tablets):
[0074]
[0075]
[0076] Prepared by usual techniques.
[0077] 3. PPD tablet prescription (1000 tablets)
[0078]
[0079] Prepared by usual techniques.
[0080] 4. Ginsenogenin mixture (1000 tablets):
[0081]
[0082] Prepared by usual techniques.
[0083] 5. Compound prescription for PPT tablets (1000 tablets)
[0084]
[0085] Prepared by usual techniques.
Embodiment 2
[0086] Embodiment two: the preparation of PPT, PPD, ginsenoside mixture capsule
[0087] 1. PPT capsule prescription (1000 capsules):
[0088] PPT 500g
[0089] Magnesium Stearate Appropriate amount
[0090] Lactose 30-120g
[0091] Prepared by usual techniques.
[0092] 2. PPD capsule prescription (1000 capsules):
[0093] PPD 500g
[0094] Starch 50-200g
[0095] Magnesium Stearate Appropriate amount
[0096] Prepared by usual techniques.
[0097] 3. Weigh 250g of PPT, and prepare hard capsules containing 250mg of PPT in each capsule with the method of prescription 1.
[0098] 4. Ginsenogenin mixture capsule prescription (1000 capsules):
[0099] Ginsenogen Mixture 500g
[0100] Magnesium Stearate Appropriate amount
[0101] Lactose 30-120g
[0102] Prepared by usual techniques.
Embodiment 3
[0103] Example Three: Preparation of PPT, PPD and Ginsenogen Mixture Injection
[0104] 1. PPT injection prescription:
[0105] PPT 250g
[0106] Tween 80 25-80g
[0107] Prepared by usual techniques. Add 1000ml of water for injection, stir to dissolve completely, filter, potting, sterilization, light inspection, packaging to make each tube containing 0.5g / 2ml of PPT.
[0108] 2. PPD injection prescription:
[0109]
[0110] Prepared by usual techniques.
[0111] 3. Prescription of ginsenogenin mixture injection: Weigh 250g of ginsenogenin mixture, and prepare it in the same way as in prescription 2, so that each tube contains 0.5g / 2ml of ginsenogenin mixture.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com