Clinical pharmacological test information processing system
An information processing system and clinical trial technology, applied in the field of clinical pharmacological trial information processing system, can solve the problems of waste of research and development investment, loss of research and development results, delay in clinical trials of drugs, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
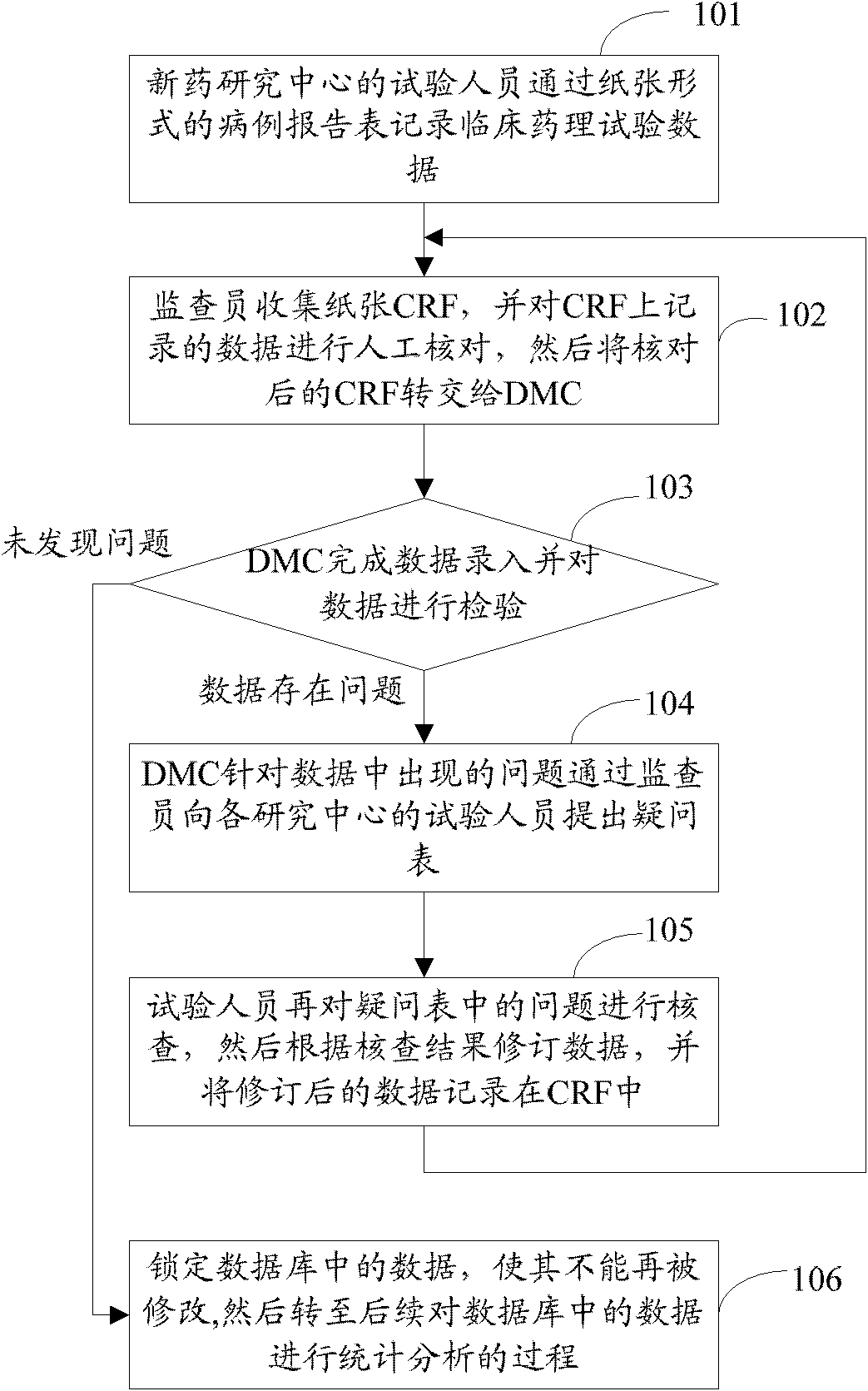
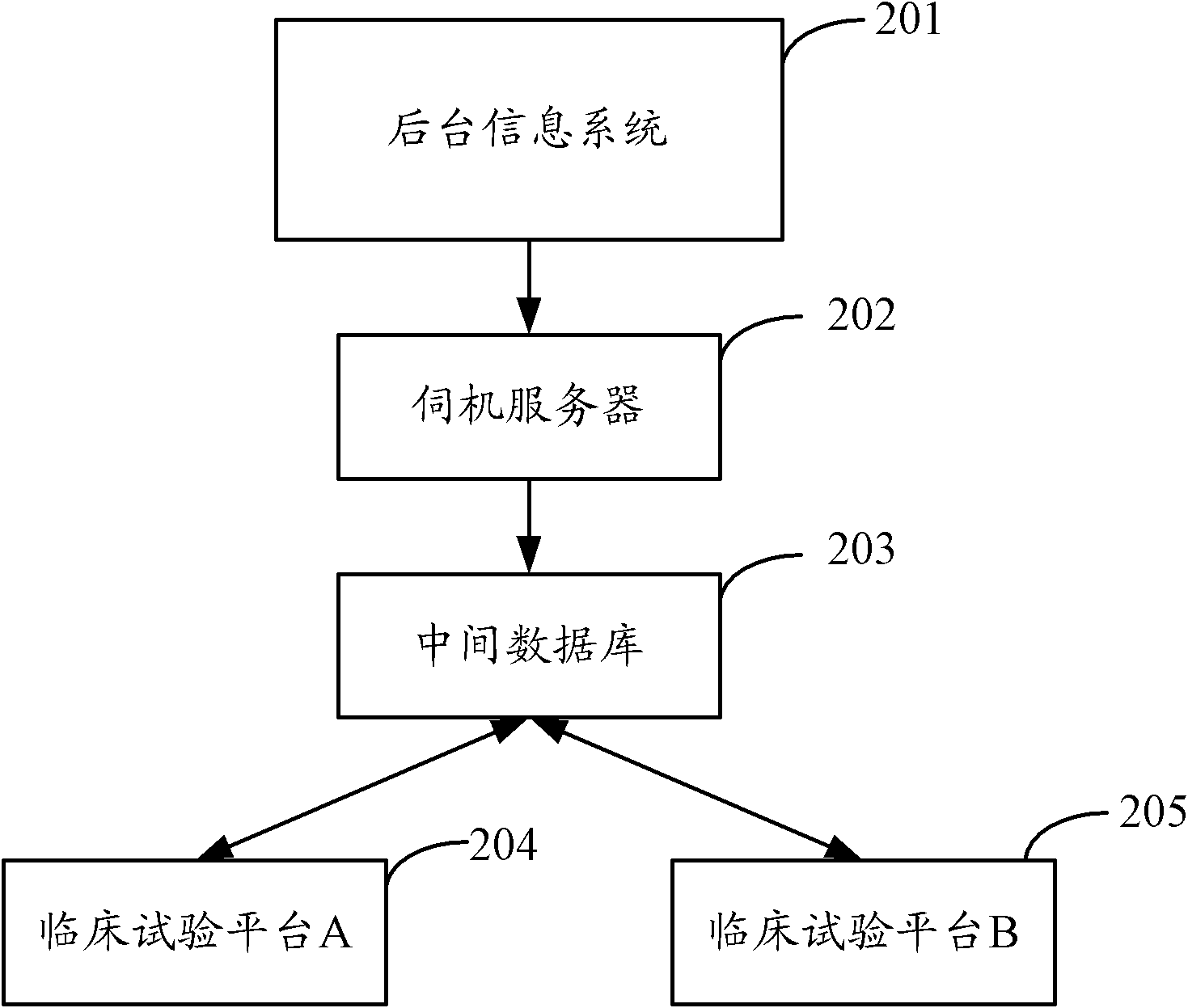
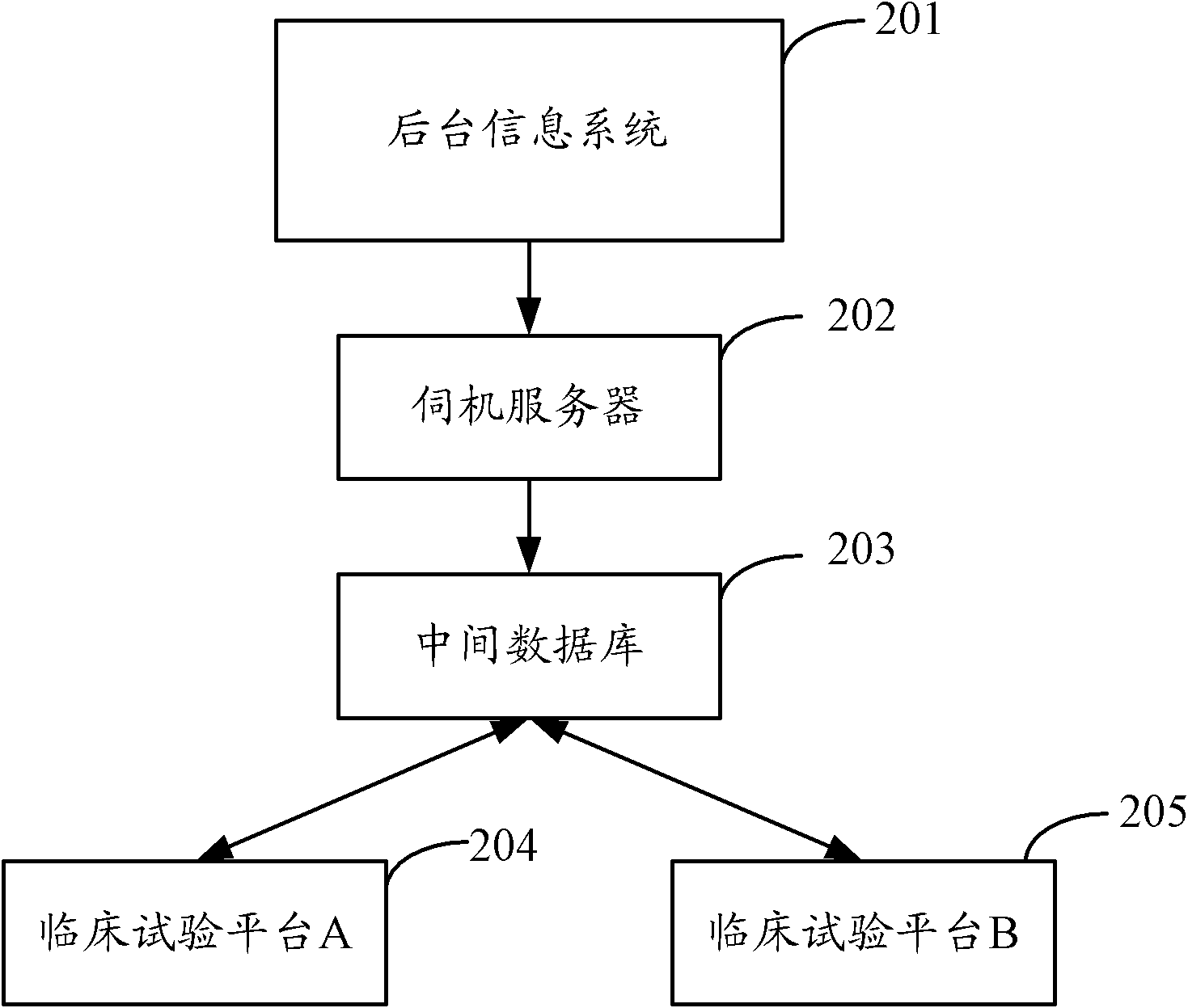
[0025] In order to make the purpose, technical solution and advantages of the present invention clearer, the present invention will be further elaborated below in conjunction with the accompanying drawings.
[0026] figure 2 Shown is a block diagram of a processing system for clinical pharmacological test information proposed by the embodiment of the present invention.
[0027] The background information system 201 is a general term for various information processing systems adopted in hospitals, including but not limited to hospital information system (Hospital Information System, HIS), laboratory information system (Laboratory Information Management System, LIS), image archiving and communication system (Picture Archiving and Communication Systems, PACS), etc.
[0028] The hospital information system HIS uses computer and communication equipment to provide various departments of the hospital with the ability to collect, store, process, extract and exchange data on patient ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com