Pharmaceutical composition
A compound and thermostatic animal technology, which is applied in the direction of drug combination, pharmaceutical formula, antineoplastic drugs, etc., can solve the problems of not indicating PDH complexes, regulating the activity of PDH complexes, etc., and achieve the effect of minimizing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0187] Chemical synthesis of thioctan
[0188] Lipoic acid derivatives (ie thioctan) CPI-613 and CPI-157 were synthesized using 6,8-dimercaptooctanoic acid as a starting material by using modified methods described in US 6,331,559B1 and US 6,951,887B2. Thioctan CPI-045 was synthesized as described in US 6,331,559B1.
[0189] The structural analysis of these three thioctans is as follows. Multiple independent syntheses of CPI-613 and CPI-157 are indistinguishable in their anticancer properties. In allogeneic transplantation ( Figure 7 ) and ATP measurements ( Figure 9 ) of CPI-613 used in the purity of more than 99%. All other preparations were greater than 98% pure.
[0190] CPI-613: 6,8-dibenzylsulfanyl octanoic acid: white crystalline solid, melting point 65-66°C (documentation 1 67.5-69°); 1 H-NMR (250MHz, CDCl 3 ): δ7.15-7.4(m, 10H), 3.66(s, 2H), 3.64(s, 2H), 2.52-2.62(m, 1H), 2.50(t, J=7.6Hz, 2H), 2.29( t, J=7.6Hz, 2H), 1.2-1.8(m, 8H); 13 C-NMR (62.9MHz, CDCl3...
Embodiment 2
[0194] Method for determining the anticancer efficacy of THIOCTAN
[0195]Cells: Human tumor cell lines were obtained from ATCC and propagated according to ATCC recommendations. Human mammary epithelial cells (HMEC), small airway epithelial cells (SAEC) and normal human epidermal keratinocytes (NHEK) primary cells were obtained from LONZA Walkersville, Inc (Walkersville, MD). Each cell line was maintained and propagated in the appropriate medium developed by the supplier and purchased from the supplier according to the supplier's instructions. The experiments reported here used normal cells at passages 3 to 6.
[0196] Tumor Growth Inhibition Study: Human BxPC-3 or AsPC-1 pancreatic tumor cells or H460 NSCLC were transplanted subcutaneously (SC) into CD1-Nu / Nu female mice. About 8-12 days later, the mice were injected intraperitoneally (IP) according to the dosage and schedule shown in the legend. Drug or vehicle was injected approximately 2 ml per 25 gm body weight. Drug ...
Embodiment 3
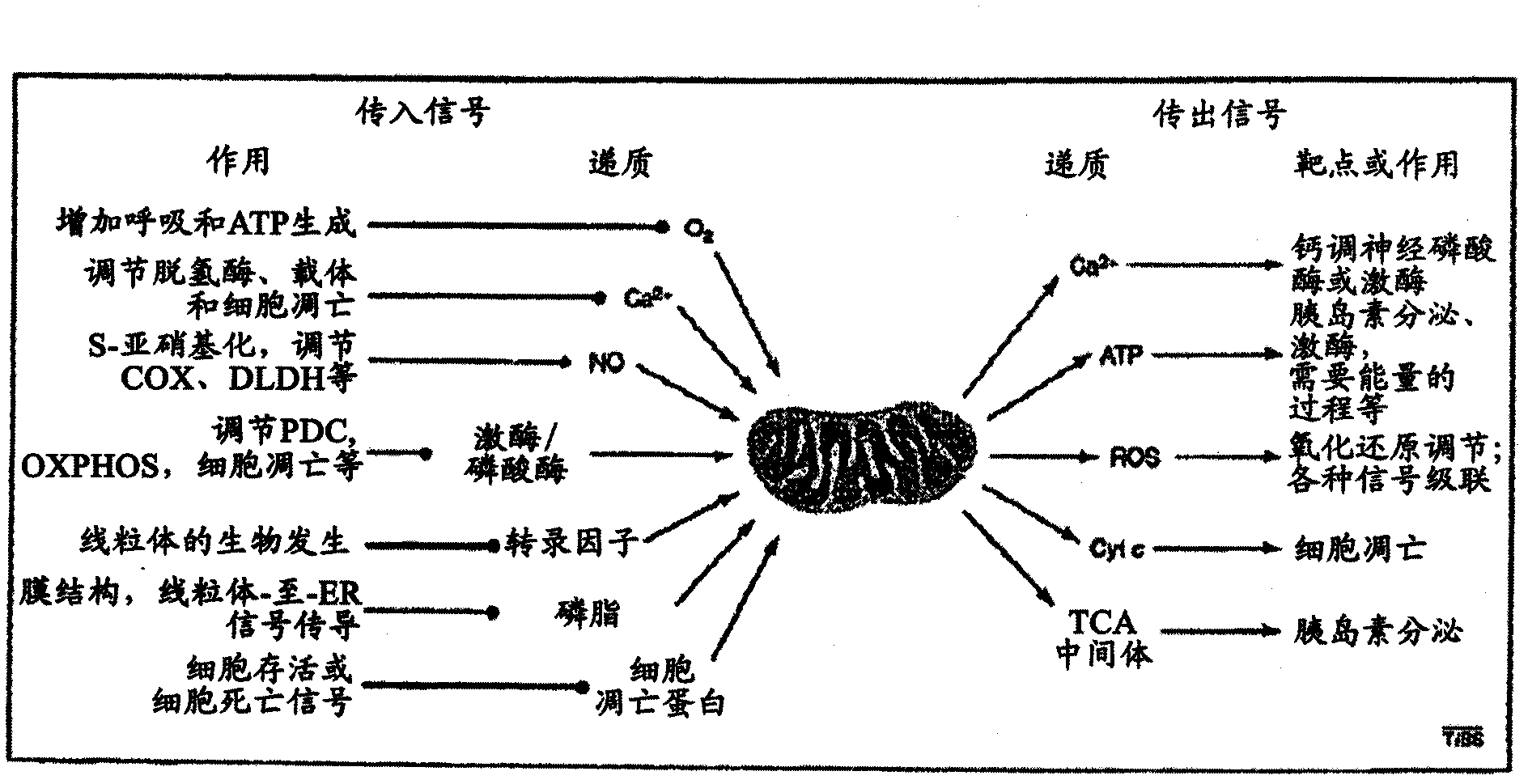
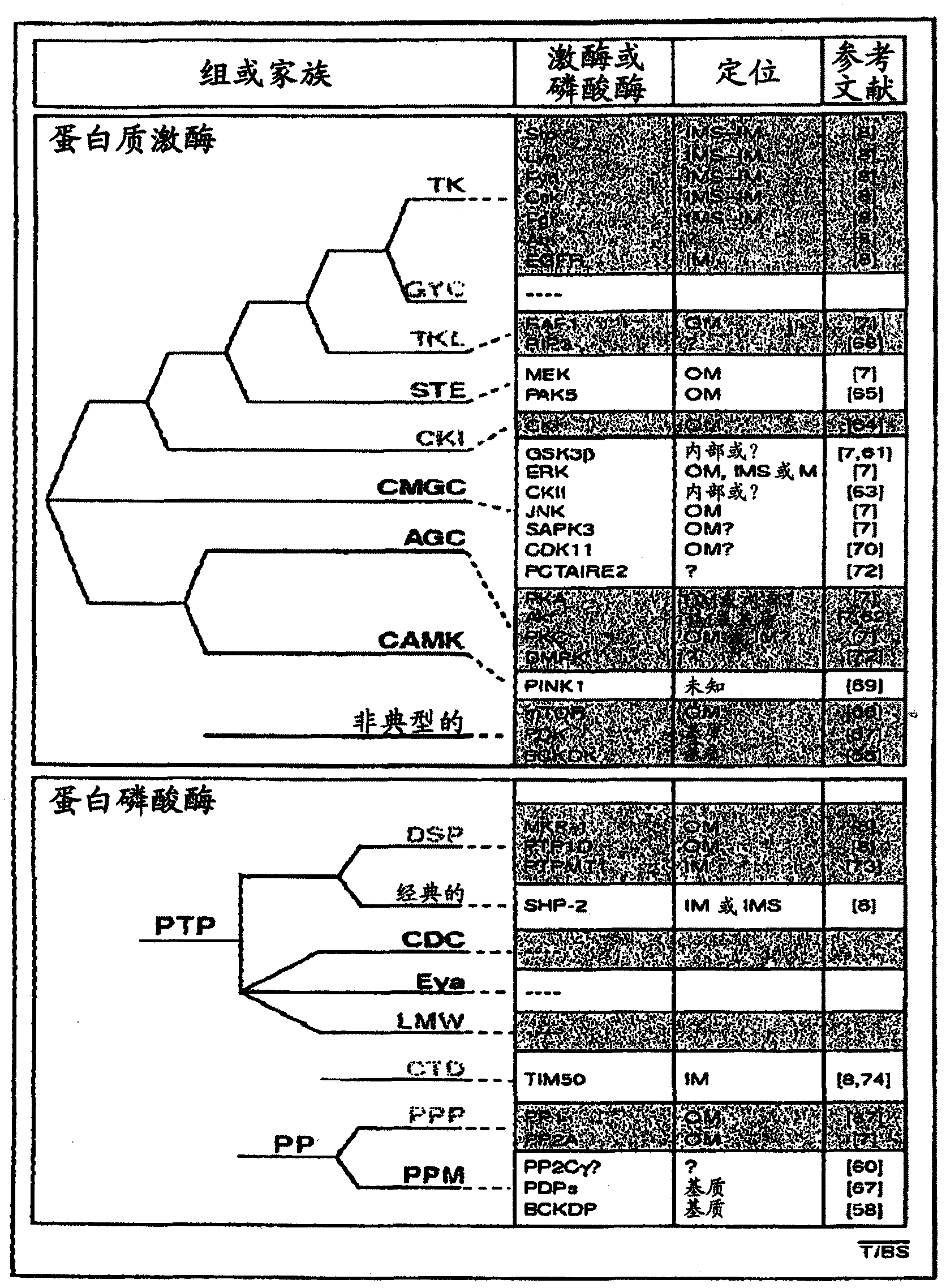
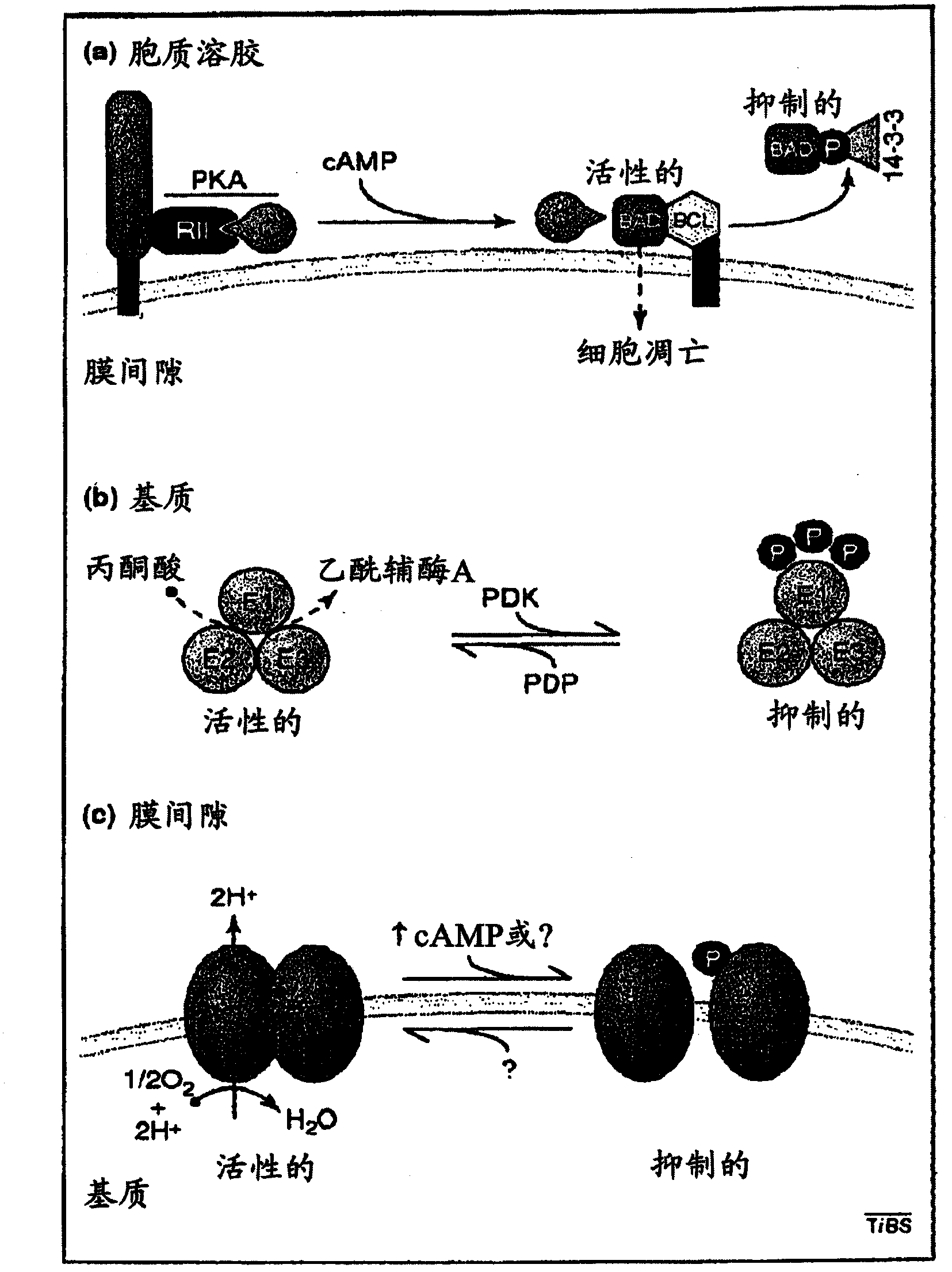
[0217] Thioctan interferes with mitochondrial membrane potential and Ca +2 intake
[0218] Substrate effect of Thioctan on ATP synthesis inhibition ( Figure 9 ) showed that the drug interferes with the TCA cycle in the mitochondrial matrix. If so, we would expect the mitochondrial membrane potential 1 May decrease at lethal threshold doses and above. Using the potential-sensitive dye TMRE, we observed the desired effect. ( Figure 13 ) With the initiation of drug treatment, the mitochondrial membrane potential decreased rapidly. The kinetics of the decrease in membrane potential closely resemble the loss of ATP synthesis in the presence of mitochondrial substrates. ( Figure 9 )
[0219] ATP reduction in mitochondria is known to trigger the uptake of Ca released from cytoplasmic reserves, including the endoplasmic reticulum +2 Equilibrium homeostatic response to uptake. Moreover, it is believed that this Ca +2 Input to the mitochondrial matrix requires the mitochon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com