Process for production of pentamethylenediamine, and process for production of polyamide resin
A kind of technology of pentamethylene diamine and pentamethylene diamine crude product, which is applied in the manufacturing field of pentamethylene diamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244]
[0245] (Thermal decomposition process)
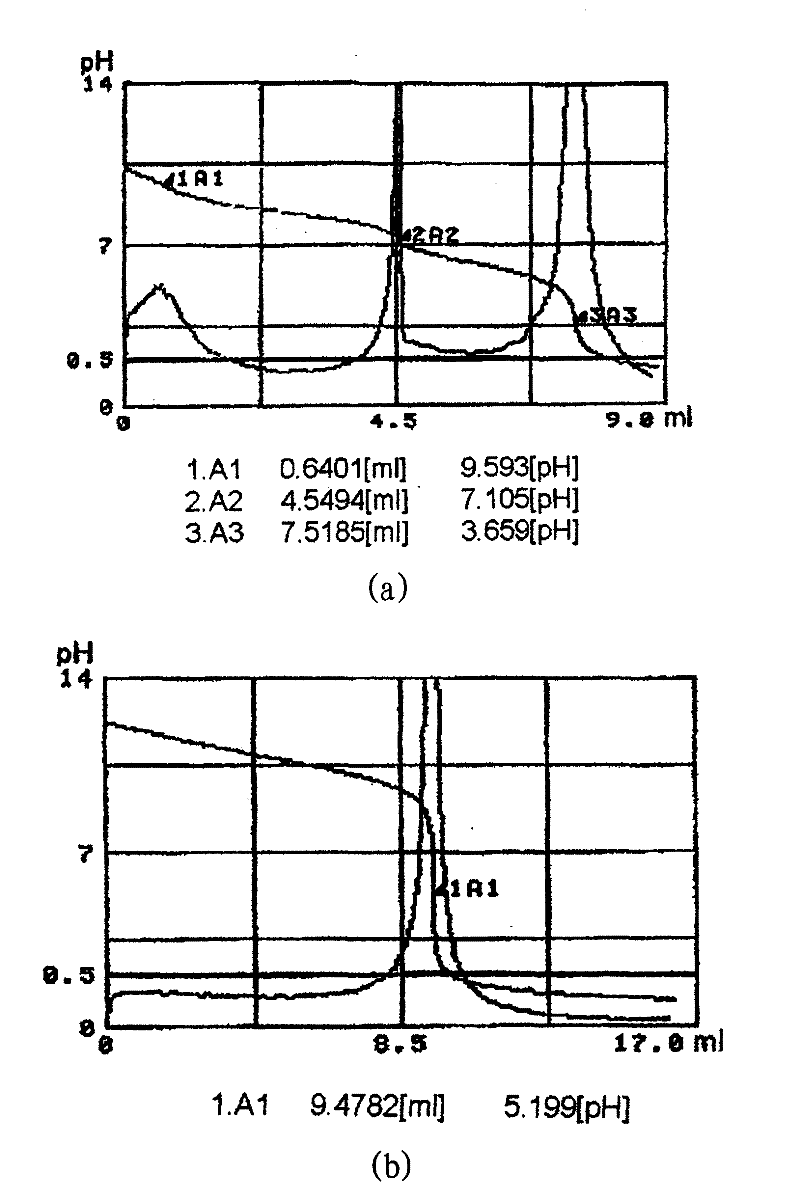
[0246] Add 5600 g of the above pentamethylene diamine carbonate aqueous solution (ii) (total pentamethylene diamine concentration 18.7% by weight) into the flask at an internal temperature of 102°C (oil bath temperature of 139°C) and normal pressure conditions When the water is recovered, it begins to decompose into crude pentamethylene diamine and carbon dioxide. After the decomposition started, the temperature was gradually increased while maintaining the normal pressure, and the decomposition was terminated when the final internal temperature reached 180°C (oil bath temperature 191°C) to obtain crude pentamethylene diamine. It should be noted that the maximum temperature of the thermal decomposition step is 180°C. The total pentamethylene diamine concentration, pentamethylene diamine concentration and pentamethylene diamine carbonate concentration in the obtained crude pentamethylene diamine were determined based on the above-...
Embodiment 2
[0262] (Thermal decomposition process and distillation process)
[0263] In the thermal decomposition step, while blowing nitrogen into the gas phase, the decomposition of the pentamethylene diamine carbonate aqueous solution (ii) was carried out, except that the same operations as in Example 1 were performed to obtain purified pentamethylene diamine 959 g (purity: 99.4% by weight). The yield was 91.0%. In addition, the maximum temperature of the internal temperature in the thermal decomposition step is 180°C. The total pentamethylene diamine concentration, pentamethylene diamine concentration and pentamethylene diamine carbonate concentration in the obtained crude pentamethylene diamine were determined based on the above-mentioned measurement method, and the concentrations were respectively 96.8 Weight%, 96.8% by weight, 0.0% by weight. According to the measurement result, in the crude pentamethylene diamine, the concentration of pentamethylene diamine relative to pentamethyl...
Embodiment 3
[0267] (Thermal decomposition process)
[0268] Add 5600 g of the above pentamethylene diamine carbonate aqueous solution (ii) (total pentamethylene diamine concentration 18.7% by weight) into the flask, at an internal temperature of 102°C (oil bath temperature of 137°C), and under normal pressure After heating and refluxing, it is decomposed into crude pentamethylene diamine and carbon dioxide. The total pentamethylene diamine concentration, pentamethylene diamine concentration and pentamethylene diamine carbonate concentration in the above crude pentamethylene diamine were determined based on the above-mentioned measurement method, and the concentrations were 21.1% by weight respectively , 14.9% by weight, 10.0% by weight. According to this measurement result, in the crude pentamethylene diamine, the concentration of pentamethylene diamine relative to pentamethylene diamine and pentamethylene diamine carbonate is 70.5 mol%.
[0269] Next, the temperature was slowly raised while...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com