Preparation method for dihydrosafrole
A technology of dihydrosafrole and piperonylcycline, applied in the direction of organic chemistry, can solve the problems of easy occurrence of side reactions, low yield, high catalyst activity, etc., and achieve the effect of mild reaction conditions, high total yield, and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
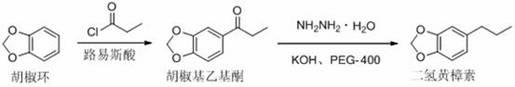
[0030] Embodiment 1, a kind of synthetic method of dihydrosafrole, take piperonylcycline and propionyl chloride as starting raw material, carry out the following steps successively:
[0031] 1), Friedel-Crafts acylation reaction of piperonyl ring:
[0032] Add 40.0g (0.294mol) of zinc chloride to 100mL (126g) of dichloroethane, control the temperature at 0~5°C, add 24.0g (0.197mol) of piperonyl dropwise, and continue at this temperature after the dropwise addition Add propionyl chloride 22.0g (0.237mol) dropwise. After the dropwise addition, continue to stir at this temperature. During the reaction, take a sample in water and take the lower organic phase for GC tracking detection until the piperonyl ring reaction is complete, about 18 hours. After stopping the reaction. Slowly add the reaction mixture into 200ml of ice water, stir and dissolve, then let it stand for stratification, wash the lower organic phase with water until it is neutral, distill off the solvent dichloroet...
Embodiment 2
[0041] Embodiment 2, a kind of synthetic method of dihydrosafrole:
[0042] Step 1) Solvent I selects tetrachloroethane (replacing ethylene dichloride in Example 1) for use, and the consumption is 100mL (160g), and the catalyst selects ferric chloride (replacing zinc chloride in Example 1), and the reaction The time was 14 hours, the amount of catalyst used (the molar ratio to the raw material was the same as in Example 1) and other operations were the same as in Example 1, and the yield of piperonyl ethyl ketone was 89.3%, and the purity was 96.8%.
[0043] Step 2) is equivalent to Example 1.
[0044] The total yield of the two-step reaction is 81.2%, and the product purity is 94.1%.
Embodiment 3
[0045] Embodiment 3, a kind of synthetic method of dihydrosafrole:
[0046] The amount of catalyst zinc chloride in step 1) is 50g (0.368mol), the amount of propionyl chloride is 25g (0.270mol), and the reaction time is 12 hours. Other operations are the same as in Example 1, and the yield of piperonyl ethyl ketone is 92.3%. , with a purity of 98.8%.
[0047] Step 2) is equivalent to Example 1.
[0048] The total yield of the two-step reaction is 84.1%, and the product purity is 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com