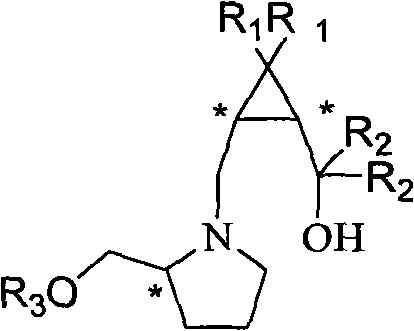
Chiral cyclopropane alkamine ligand compound, and preparation and application thereof
A technology for cyclopropaneamino and ligand compounds, which is applied to chiral cyclopropaneamino alcohol ligand compounds and the fields of preparation and application thereof, can solve problems such as the limitation of ligand development, and achieve the effects of high stereoselectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of (1R, 3S)-3-formyl-2,2-dimethylcyclopropanecarboxylic acid methyl ester
[0031] First add 250mL of methanol into a 500mL four-neck flask, then add 42.6g (300mmol) of carvalolactone, 10g (58mmol) of p-toluenesulfonic acid, stir rapidly and raise the temperature to reflux for 5h. Heating was stopped, 10 g (120 mmol) of sodium acetate was added, and the methanol was spun off under reduced pressure. 250 mL of n-hexane was added to the residue, the organic phase was washed three times with 100 mL of water, and then the organic phase was spun off. Add 200 mL of 0.5% oxalic acid aqueous solution to the residue, stir rapidly at room temperature for 10 h, extract three times with 100 mL of ether, combine the organic phases, anhydrous Na 2 SO 4 Dried and spin-dried to obtain 31 g of light yellow oil, which was directly used in the next reaction without purification.
Embodiment 2
[0032] Embodiment 2: Preparation of (1R, 3S)-3-(prolinol-1-methyl)-2,2-dimethylcyclopropanecarboxylic acid methyl ester
[0033] First add 80mL of methanol to a 250mL four-necked bottle, add 8.08g (80mmol) (or D-prolinol), slowly add 1mL (20mmol) of concentrated sulfuric acid dropwise, and then add 3.12g (40mmol) of caronaldehyde ester and 2g (32mmol) NaBH 3 CN. The above solution was stirred rapidly at room temperature for 16 hours, then concentrated hydrochloric acid was added dropwise to make the pH10, extract three times with 15 mL of diethyl ether, combine the organic phases, anhydrous MgSO 4 Dry and purify.
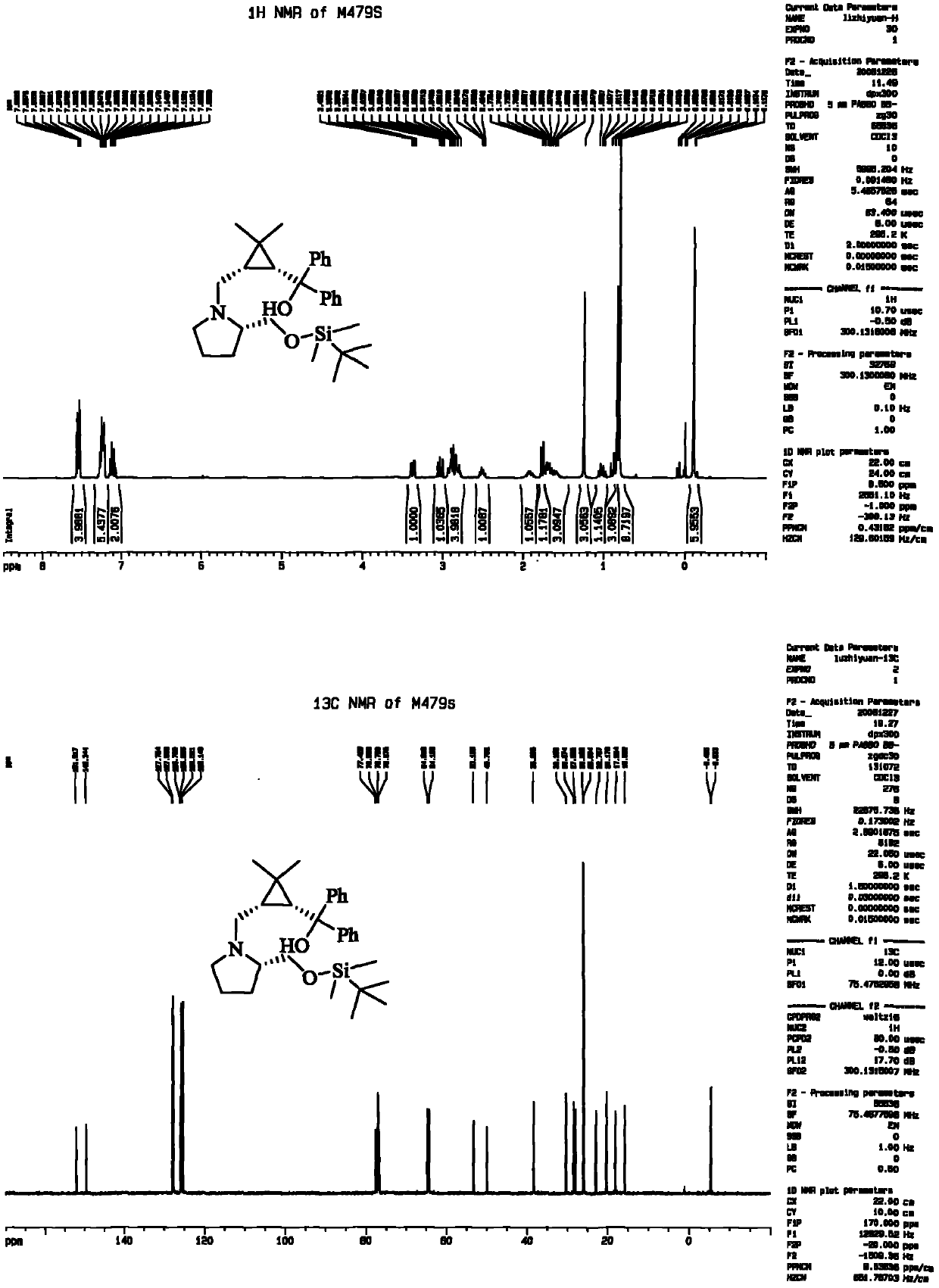
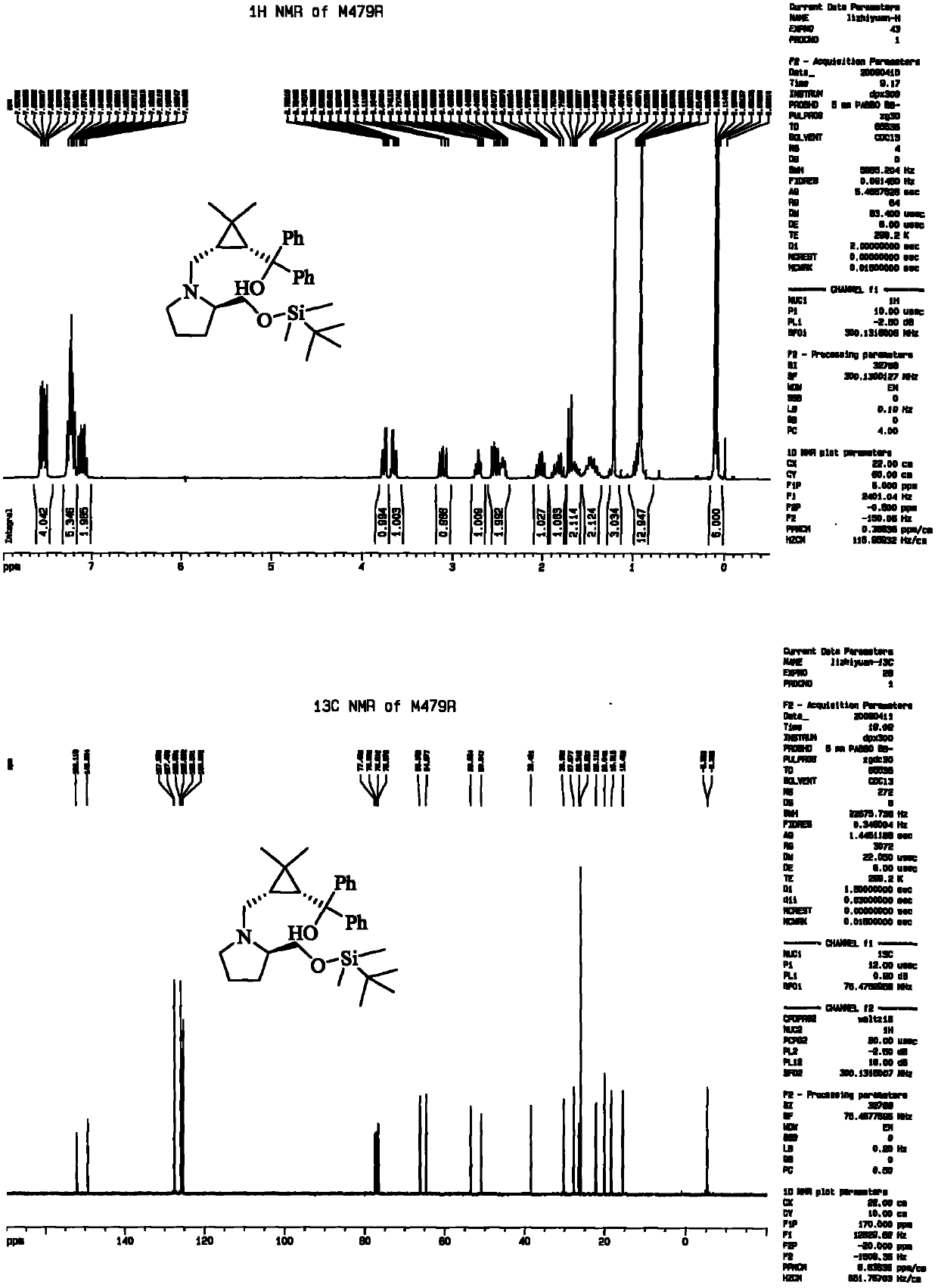
[0034] 5a: Column chromatography: anhydrous diethyl ether, a light yellow oily liquid was obtained with a yield of 78%. [α] D 20 =-49.1 (c=1.46, CHCl 3 ); IR (neat) 3427, 2952, 2875, 1727, 1438, 1385, 1277, 1178, 1140, 1091, 1045cm -1 ; 1 H NMR (300MHz, CDCl 3 ): δ3.69-3.62(m, 4H), 3.39-3.35(m, 1H), 3.20-3.07(m, 2H), 3.86(br, 1H), 2.66-2.59(m, 2H), 2.29-2.2...
Embodiment 3
[0036] Example 3: Preparation of (1R, 3S)-3-(prolinol-1-methyl)-2,2-dimethylcyclopropanediphenylmethanol
[0037] Under the protection of nitrogen, first add 1.2g (5mmol) prolinol cyclopropane ester, then add anhydrous THF 5mL, slowly add 10mL PhMgCl (2mol / L) dropwise under ice bath, after the dropwise addition is completed, rise to room temperature and continue the reaction for 12h. After the reaction was complete, 5 mL of saturated NH 4 Cl, extracted three times with ether 10mL and combined organic phase, anhydrous Na 2 SO 4 After drying, the solvent was removed to obtain a white solid, which was purified by recrystallization.
[0038] 6a: white solid, yield 82%, mp 89-91 °C, [α] D 20 =+37.1 (c=2.48, CHCl 3 ); IR (KBr) 3413, 3057, 3010, 2931, 2873, 2818, 1600, 1491, 1447, 1413, 1374, 1304, 1077, 1028, 752, 702cm -1 ; 1 H NMR (300MHz, CDCl 3 ): δ7.58-7.49(m, 4H), 7.32-7.24(m, 4H), 7.18-7.15(m, 2H), 5.50(br, 1H), 3.28(d, J=4Hz, 1H), 3.07 -2.93(m, 2H), 2.80-2.74(m, 2H)...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap