Application of brusatol as chemotherapeutic drug synergist
A technique for nucleoside and a chemotherapeutic agent, which is applied in the field of nucleoside as a synergist of chemotherapeutic agents to achieve the effect of increasing growth inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
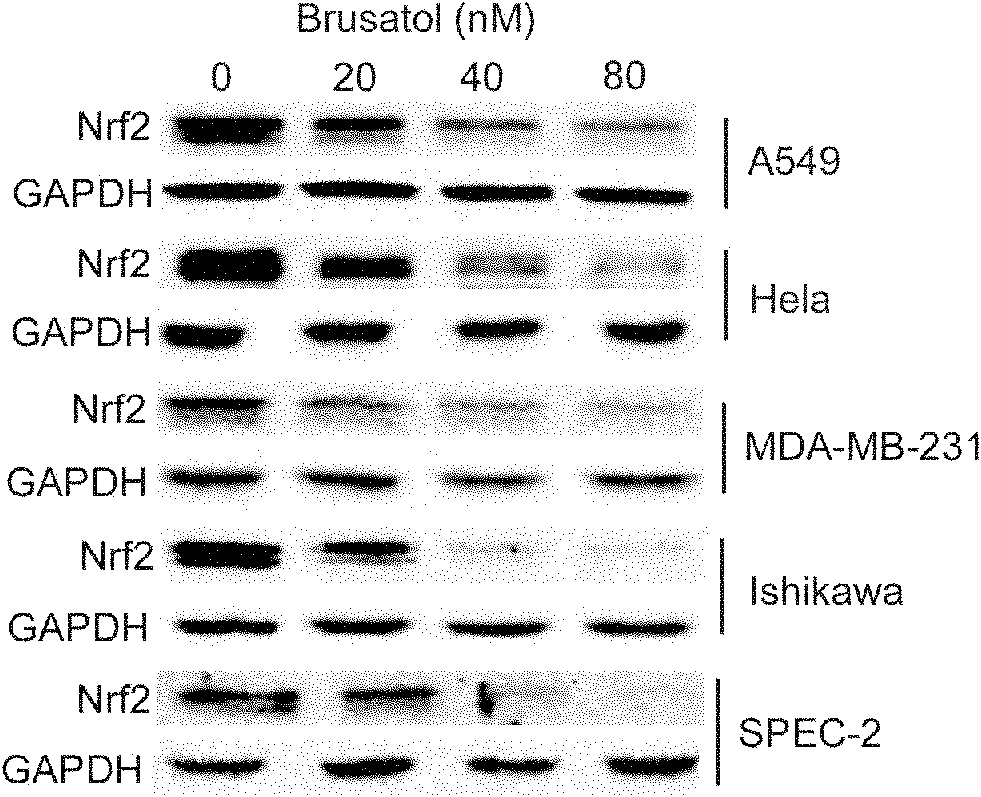
[0027] Example 1: Determination of Nrf2 protein expression in non-small cell lung cancer A549, cervical cancer Hela, endometrial cancer Ishikawa and Spec-2, and breast cancer MDA-MB-231 cell lines
[0028] Experimental materials: Non-small cell lung cancer A549, cervical cancer Hela, endometrial cancer Ishikawa and Spec-2, breast cancer MDA-MB-231 cell lines were purchased from ATCC in the United States; bruceicol was isolated from Brucea javanica, NMR and MS Determine the structure; Nrf2 and GAPDH antibodies were purchased from Santa Cruz; cisplatin, carboplatin, etoposide, 5-FU and paclitaxel were purchased from Sigma.
[0029] experimental method:
[0030] (1) Cell treatment: Cells were treated with 1×10 6 The density of cells per well was seeded in 6-well plates, and treated with cholepicrol DMSO solution for 4 hours.
[0031] (2) Cell lysis: After the cell treatment is completed, wash the cells twice with ice PBS to remove residual drugs, add SDS cell lysate 100 μl / well...
Embodiment 2
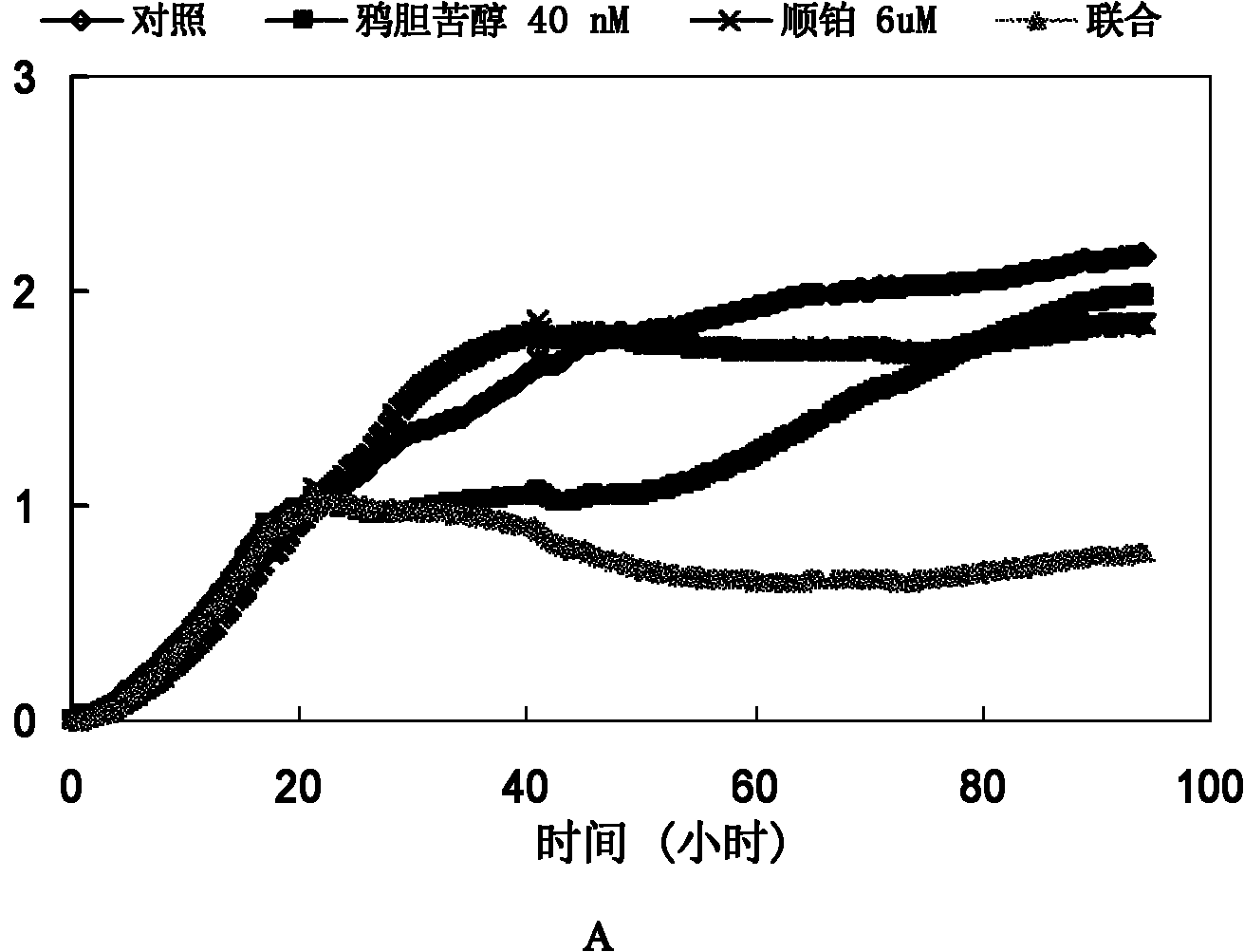
[0034] Example 2: Brucelipidol increases the effect of cisplatin, carboplatin, 5-FU, etoposide and paclitaxel on A549 cells
[0035] Experimental materials: the sources of cell lines, drugs, and culture medium are the same as those in Example 1.
[0036] Instrument: Roche Xcelligence cell real-time detection system.
[0037] Experimental method: Cells were inoculated in Xcelligence special 16-well plate at a density of 8000 per well. After the cells had adhered to the wall for 16-24 hours, they were pretreated with cholepicol for 4 hours, and then chemotherapeutic drugs were added for co-cultivation. The cell growth index was recorded for 30 minutes and recorded for 72 hours.
[0038] Experimental results: The application of bruceicol alone temporarily inhibited the cells. With the prolongation of the treatment time, the cells in the bruceicol treatment group gradually resumed growth, while the cell growth index of the combined drug group was significantly smaller than that o...
Embodiment 3
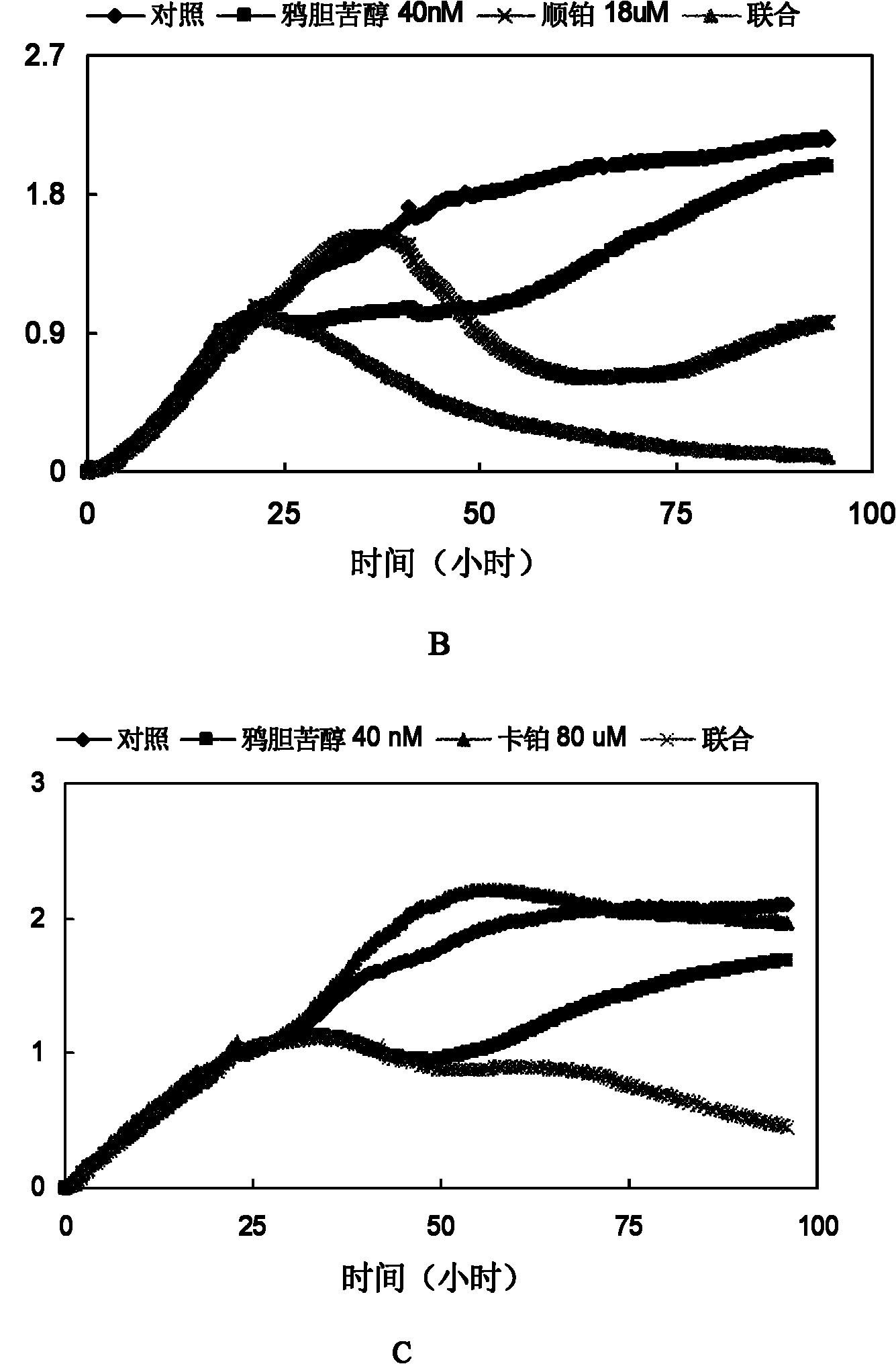
[0039] Example 3: Brucelipidol can increase the effect of cisplatin and carboplatin on Hela cells
[0040] Experimental material and experimental method are the same as embodiment 2.
[0041] Experimental results: Brucelipidin showed a synergistic effect with chemotherapeutic drugs in Hela cells, see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com