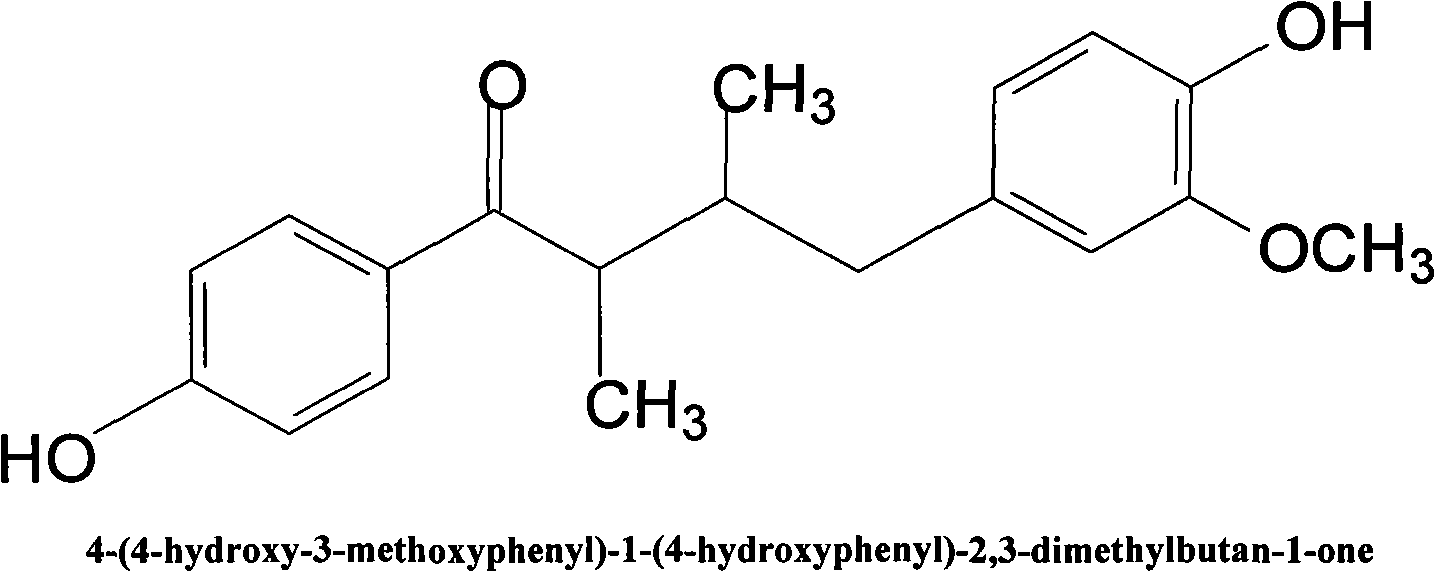
4-(4-hydroxy-3-methoxyphenyl)-1-(4-phenyl)-2, 3-dimethyl butyl-1-ketone in loropetalum leaves and application thereof
A technology of dimethylbutyl and methoxyphenyl, which is applied in the separation/purification of ketones, carbonyl compounds, skin diseases, etc., can solve the problems that have not been reported in the literature, and achieve a clear and easy-to-use material basis. Accepted and beneficial to the effect of exporting foreign exchange
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
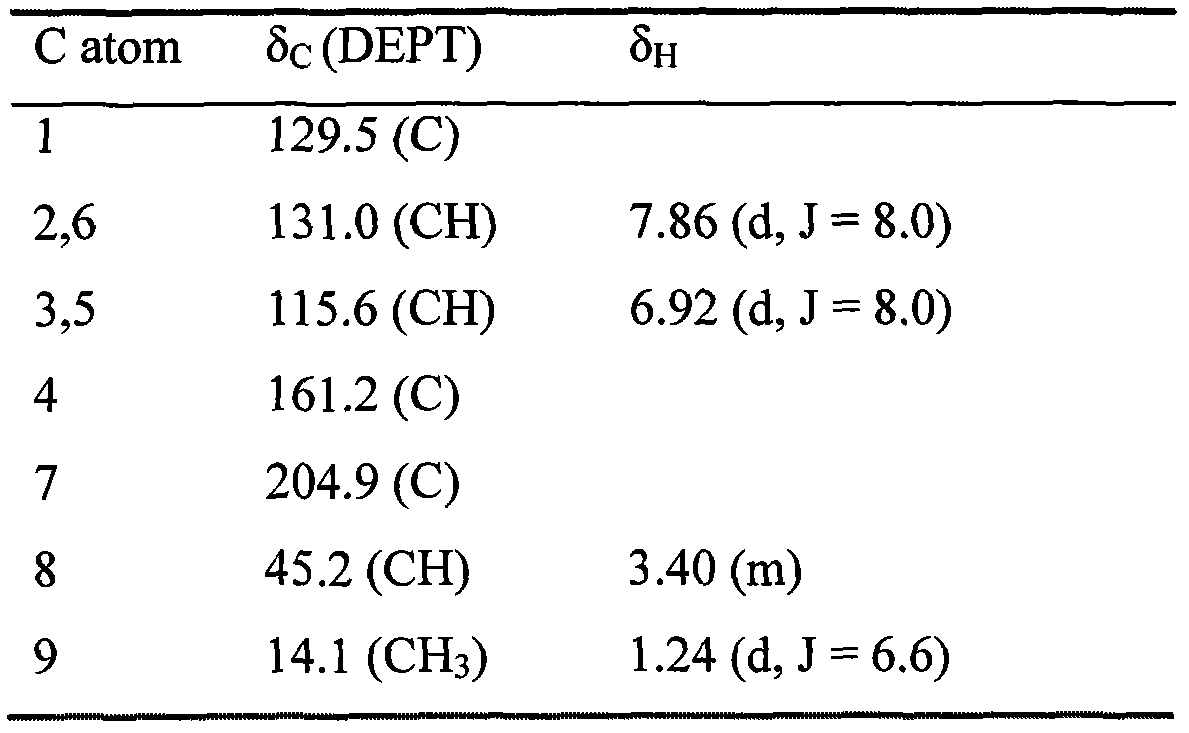
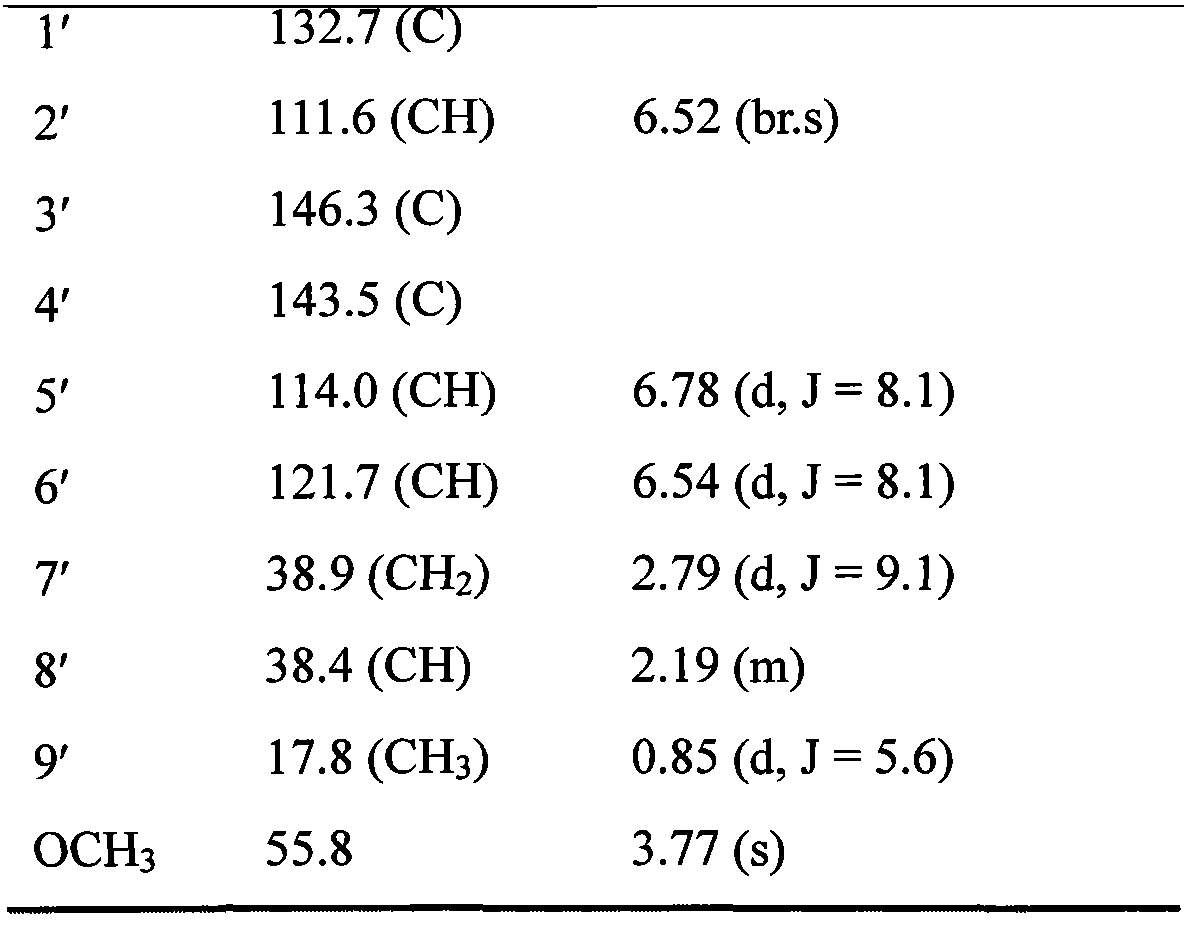
[0017] Take 200kg of loropetalum leaves, add 90% ethanol to reflux and extract twice, each time for 1 hour, add 1000kg90% ethanol for the first time, add 800kg90% ethanol for the second time, filter, combine the extracts, and recover ethanol from the extracts and concentrated to a dilute extract with a relative density of 1.05, pass the dilute extract through D101 macroporous adsorption resin, first elute with 30% ethanol until colorless, discard the 30% ethanol eluate, and then use 90% ethanol The ethanol was eluted to colorless, and the 90% ethanol eluate was collected, and the ethanol was recovered to obtain the extract. The extract was mixed with silica gel and added to a silica gel column, chloroform-methanol gradient elution, the eluate was collected, identified by thin-layer chromatography, combined, passed through a SephadexLH-20 gel column, and eluted with methanol. Collect eluate, identify by thin layer chromatography, combine, recover methanol, pass through RP-18 re...
Embodiment 2
[0028] Take the 4-(4-hydroxy-3-methoxyphenyl)-1-(4-hydroxyphenyl)-2,3-dimethylbutyl-1-one and add it in a ratio of 1:2 Dextrin, mixed evenly, adopts the existing hard capsule preparation process to obtain 4-(4-hydroxy-3-methoxyphenyl)-1-(4-hydroxyphenyl)-2,3-dimethyl The drug in the form of hard capsules of butyl-1-one.
Embodiment 3
[0030] Take the 4-(4-hydroxy-3-methoxyphenyl)-1-(4-hydroxyphenyl)-2,3-dimethylbutyl-1-one and add it in a ratio of 1:3 Vegetable oil, mixed evenly, using gelatin as the capsule shell material, adopting the existing soft capsule preparation process to obtain 4-(4-hydroxyl-3-methoxyphenyl)-1-(4-hydroxyphenyl)- 2,3-Dimethylbutyl-1-one is a drug in the form of soft capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com