Kit for rapidly and synchronously detecting nucleic acids of influenza virus A
An influenza virus, simultaneous detection technology, applied in recombinant DNA technology, microbial determination/inspection, DNA/RNA fragments, etc., can solve problems such as increased workload and detection cost, large sample demand, and easy missed detection. To achieve the effect of improving specificity, high sensitivity and improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The composition and preparation of embodiment 1 type A influenza virus H1, H3, H5 and H9 subtype rapid simultaneous detection kit:
[0045] 1. Reagent composition: Trizol RNA lysate is a product of Invitrogen; Taq DNA polymerase (2U / μl), dNTPs (10mM), Mg 2+ (1.5mM), M-MLV enzyme (200U / μl) are Promega products; detection primers: H1, H3, H5 and H9 subtype influenza virus PCR primers and conserved M gene primers mixed in equal proportions; reverse transcription primers : 12 conserved nucleotides at the 3′ end of the influenza A virus genome; all primers were synthesized by Shanghai Sangon Bioengineering Company; H1, H3, H5, H9 subtypes and M positive quality control plasmids were constructed by our laboratory, save;
[0046] The relevant primer sequences are as follows:
[0047] H1 upstream primer sequence: GCCATTGCCGGTTTCATTG
[0048] Downstream primer sequence: GAAGCTGATTGCCCCCA
[0049] H3 upstream primer sequence: GGGAATGGTTGYTTCAARATATAC
[0050] Downstream ...
Embodiment 2
[0086] Example 2 Method for using the Rapid Synchronous Detection Kit for Type A Influenza Virus H1, H3, H5 and H9 Subtypes
[0087] 1. Sample processing
[0088] Specimens involved in the detection of influenza A virus include respiratory samples (nasal / pharyngeal swabs, sputum, nasopharyngeal aspirate, bronchoalveolar lavage fluid, etc.), serum in the acute phase, serum in the recovery phase, thoracentesis, etc.; Specimens also involve various tissues.
[0089] (1) If the sample contains a small amount of mucus (nasal / pharyngeal swab, nasopharyngeal aspirate, bronchoalveolar lavage fluid, etc.), put the sample in a centrifuge at 4°C and 2000rpm for 20 minutes to remove impurities. After centrifugation, gently open the centrifuge tube in a safety cabinet, pipette the supernatant into an EP tube for RNA extraction.
[0090] (2) If the sample liquid contains a large amount of mucus (sputum, etc.), add 1% trypsin solution (pH7.6) at a volume ratio of 1:1, digest at 25°C for 15-3...
Embodiment 3
[0106] Example 3 Application of Rapid Synchronous Detection Kit for Type A Influenza Virus H1, H3, H5 and H9 Subtypes
[0107] In the following experiments, Y is A and R is C in the degenerate primers used.
[0108] 1. Sensitivity test
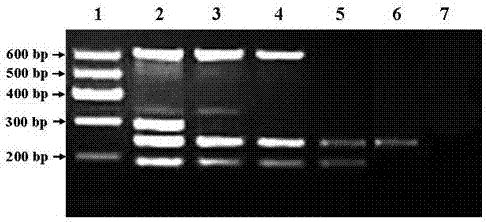
[0109] 10-fold serial dilutions of influenza A virus H1N1, H3N2, H5N1, and H9N2 subtypes 0 ~1.0×10 8 copies / ml) as a template, added to the PCR reaction solution of the detection kit, carried out the amplification reaction on the PCR instrument according to the amplification reaction conditions during the sample detection, and the PCR amplification products were analyzed by 3% agarose gel electrophoresis. It has been confirmed by repeated experiments for more than 3 times that the sensitivity of the kit for detecting H1 and H9 subtype influenza viruses is 10 copies / μl; the sensitivity for detecting H3 and H5 subtype influenza viruses is both 100 copies / μl.
[0110] 2. Specificity test
[0111] Influenza A virus H1, H3, H5 and H9 subtype ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com